Central-to-Helical-to-Axial-to-Central Transfer of Chirality with a Photoresponsive Catalyst
- PMID: 30458108
- PMCID: PMC6326533
- DOI: 10.1021/jacs.8b10816
Central-to-Helical-to-Axial-to-Central Transfer of Chirality with a Photoresponsive Catalyst
Abstract
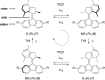
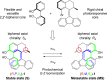
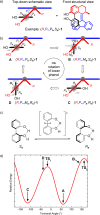
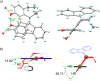
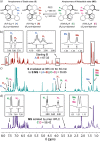
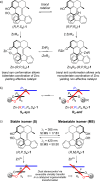
Recent advances in molecular design have displayed striking examples of dynamic chirality transfer between various elements of chirality, e.g., from central to either helical or axial chirality and vice versa. While considerable progress in atroposelective synthesis has been made, it is intriguing to design chiral molecular switches able to provide selective and dynamic control of axial chirality with an external stimulus to modulate stereochemical functions. Here, we report the synthesis and characterization of a photoresponsive bis(2-phenol)-substituted molecular switch 1. The unique design exhibits a dynamic hybrid central-helical-axial transfer of chirality. The change of preferential axial chirality in the biaryl motif is coupled to the reversible switching of helicity of the overcrowded alkene core, dictated by the fixed stereogenic center. The potential for dynamic control of axial chirality was demonstrated by using ( R)-1 as switchable catalyst to direct the stereochemical outcome of the catalytic enantioselective addition of diethylzinc to aromatic aldehydes, with successful reversal of enantioselectivity for several substrates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Eliel E. L.; Wilen S. H.. Stereochemistry of Organic Compounds; Wiley: New York, 1994.
-
- Yamamoto H.; Carreira E. M.. Comprehensive Chirality; Elsevier Science: Oxford, 2012.
-
- Bentley R. In Encyclopedia of Molecular Cell Biology and Molecular Medicine; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

