Oxidative Stress in Cells with Extra Centrosomes Drives Non-Cell-Autonomous Invasion
- PMID: 30458137
- PMCID: PMC6251975
- DOI: 10.1016/j.devcel.2018.10.026
Oxidative Stress in Cells with Extra Centrosomes Drives Non-Cell-Autonomous Invasion
Abstract
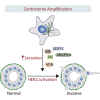
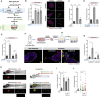
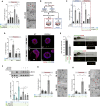
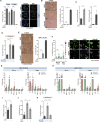
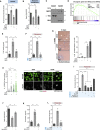
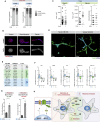
Centrosomal abnormalities, in particular centrosome amplification, are recurrent features of human tumors. Enforced centrosome amplification in vivo plays a role in tumor initiation and progression. However, centrosome amplification occurs only in a subset of cancer cells, and thus, partly due to this heterogeneity, the contribution of centrosome amplification to tumors is unknown. Here, we show that supernumerary centrosomes induce a paracrine-signaling axis via the secretion of proteins, including interleukin-8 (IL-8), which leads to non-cell-autonomous invasion in 3D mammary organoids and zebrafish models. This extra centrosomes-associated secretory phenotype (ECASP) promotes invasion of human mammary cells via HER2 signaling activation. Further, we demonstrate that centrosome amplification induces an early oxidative stress response via increased NOX-generated reactive oxygen species (ROS), which in turn mediates secretion of pro-invasive factors. The discovery that cells with extra centrosomes can manipulate the surrounding cells highlights unexpected and far-reaching consequences of these abnormalities in cancer.
Keywords: HER2; IL-8; ROS; cancer; centrosome amplification; invasion; paracrine signaling; secretion; senescence.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Arnandis T., Godinho S.A. Studying centrosome function using three-dimensional cell cultures. Methods Cell Biol. 2015;129:37–50. - PubMed
-
- Benoy I.H., Salgado R., Van Dam P., Geboers K., Van Marck E., Scharpe S., Vermeulen P.B., Dirix L.Y. Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clin. Cancer Res. 2004;10:7157–7162. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

