Sex Chromosome Effects on Male-Female Differences in Mammals
- PMID: 30458153
- PMCID: PMC6264392
- DOI: 10.1016/j.cub.2018.09.018
Sex Chromosome Effects on Male-Female Differences in Mammals
Abstract
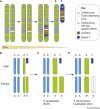
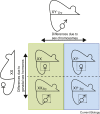
Fundamental differences exist between males and females, encompassing anatomy, physiology, behaviour, and genetics. Such differences undoubtedly play a part in the well documented, yet poorly understood, disparity in disease susceptibility between the sexes. Although traditionally attributed to gonadal sex hormone effects, recent work has begun to shed more light on the contribution of genetics - and in particular the sex chromosomes - to these sexual dimorphisms. Here, we explore the accumulating evidence for a significant genetic component to mammalian sexual dimorphism through the paradigm of sex chromosome evolution. The differences between the extant X and Y chromosomes, at both a sequence and regulatory level, arose across 166 million years. A functional result of these differences is cell autonomous sexual dimorphism. By understanding the process that changed a pair of homologous ancestral autosomes into the extant mammalian X and Y, we believe it easier to consider the mechanisms that may contribute to hormone-independent male-female differences. We highlight key roles for genes with homologues present on both sex chromosomes, where the X-linked copy escapes X chromosome inactivation. Finally, we summarise current experimental paradigms and suggest areas for developments to further increase our understanding of cell autonomous sexual dimorphism in the context of health and disease.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Randall J.C., Winkler T.W., Kutalik Z., Berndt S.I., Jackson A.U., Monda K.L., Kilpeläinen T.O., Esko T., Mägi R., Li S. Sex-stratified genome-wide association studies including 270,000 individuals show sexual dimorphism in genetic loci for anthropometric traits. PLoS Genet. 2013;9:e1003500. - PMC - PubMed
-
- Plavcan J.M. Sexual dimorphism in primate evolution. Am. J. Phys. Anthropol. 2002;116:25–53. - PubMed
-
- Arnold A.P. Sex chromosomes and brain gender. Nat. Rev. Neurosci. 2004;5:701–708. - PubMed
-
- Burgoyne P.S., Thornhill A.R., Boudrean S.K., Darling S.M., Bishop C.E., Evans E.P., Capel B., Mittwoch U. The genetic basis of XX-XY differences present before gonadal sex differentiation in the mouse [and Discussion] Philos. Trans. R. Soc. Lond. B Biol. Sci. 1995;350:253–261. - PubMed
-
- Cui W., Ma C.-X., Tang Y., Chang V., Rao P.V., Ariet M., Resnick M.B., Roth J. Sex differences in birth defects: A study of opposite-sex twins. Birth Defect Res. A Clin. Mol. Teratol. 2005;73:876–880. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

