Genetic suppression of cryoprotectant toxicity
- PMID: 30458175
- PMCID: PMC7001869
- DOI: 10.1016/j.cryobiol.2018.11.003
Genetic suppression of cryoprotectant toxicity
Abstract
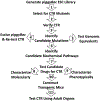
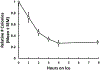
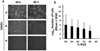
We report here a new, unbiased forward genetic method that uses transposon-mediated mutagenesis to enable the identification of mutations that confer cryoprotectant toxicity resistance (CTR). Our method is to select for resistance to the toxic effects of M22, a much-studied whole-organ vitrification solution. We report finding and characterizing six mutants that are resistant to M22. These mutants fall into six independent biochemical pathways not previously linked to cryoprotectant toxicity (CT). The genes associated with the mutations were Gm14005, Myh9, Nrg2, Pura, Fgd2, Pim1, Opa1, Hes1, Hsbp1, and Ywhag. The mechanisms of action of the mutations remain unknown, but two of the mutants involve MYC signaling, which was previously implicated in CT. Several of the mutants may up-regulate cellular stress defense pathways. Several of the M22-resistant mutants were also resistant to dimethyl sulfoxide (Me2SO), and many of the mutants showed significantly improved survival after freezing and thawing in 10% (v/v) Me2SO. This new approach to overcoming CT has many advantages over alternative methods such as transcriptomic profiling. Our method directly identifies specific genetic loci that unequivocally affect CT. More generally, our results provide the first direct evidence that CT can be reduced in mammalian cells by specific molecular interventions. Thus, this approach introduces remarkable new opportunities for pharmacological blockade of CT.
Keywords: Cryoprotectant toxicity resistance; Cryoprotective mutants; Forward genetics; Freezing injury; Mice; Mutant selection; Stem cells; Stress resistance; Transposons.
Copyright © 2018. Published by Elsevier Inc.
Figures

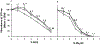

Similar articles
-
Cryoprotectant toxicity in Caenorhabditis elegans.Cryobiology. 2019 Feb;86:71-76. doi: 10.1016/j.cryobiol.2018.12.002. Epub 2018 Dec 4. Cryobiology. 2019. PMID: 30527584
-
Potential of fructans as natural cryoprotectant agents in plant cryopreservation: concept validation on Arabidopsis thaliana L.Cryo Letters. 2024 Jul-Aug;45(4):221-230. Cryo Letters. 2024. PMID: 38809786
-
Residual ethylene glycol and dimethyl sulphoxide concentration in human ovarian tissue during warming/thawing steps following cryopreservation.Reprod Biomed Online. 2017 Sep;35(3):311-313. doi: 10.1016/j.rbmo.2017.05.016. Epub 2017 Jun 6. Reprod Biomed Online. 2017. PMID: 28645837
-
Cryopreservation of animal and human embryos by vitrification.Reprod Biomed Online. 2004 Aug;9(2):164-70. doi: 10.1016/s1472-6483(10)62125-6. Reprod Biomed Online. 2004. PMID: 15333245 Review.
-
Current status of vitrification of embryos and oocytes in domestic animals: ethylene glycol as an emerging cryoprotectant of choice.Jpn J Vet Res. 1998 Feb;45(4):183-91. Jpn J Vet Res. 1998. PMID: 9553322 Review.
Cited by
-
Natural Cryoprotective and Cytoprotective Agents in Cryopreservation: A Focus on Melatonin.Molecules. 2022 May 19;27(10):3254. doi: 10.3390/molecules27103254. Molecules. 2022. PMID: 35630729 Free PMC article. Review.
-
Principles of Ice-Free Cryopreservation by Vitrification.Methods Mol Biol. 2021;2180:27-97. doi: 10.1007/978-1-0716-0783-1_2. Methods Mol Biol. 2021. PMID: 32797408
-
Chemical approaches to cryopreservation.Nat Rev Chem. 2022 Aug;6(8):579-593. doi: 10.1038/s41570-022-00407-4. Epub 2022 Jul 18. Nat Rev Chem. 2022. PMID: 37118007 Review.
-
Cryopreservation method for Drosophila melanogaster embryos.Nat Commun. 2021 Apr 23;12(1):2412. doi: 10.1038/s41467-021-22694-z. Nat Commun. 2021. PMID: 33893303 Free PMC article.
-
Chemical approaches to cryopreservation.Nat Rev Chem. 2022;6(8):579-593. doi: 10.1038/s41570-022-00407-4. Epub 2022 Jul 18. Nat Rev Chem. 2022. PMID: 35875681 Free PMC article. Review.
References
-
- Anonymous, Buying time for transplants. Nature Biotechnology, 2017. 35: p. 801. - PubMed
-
- Arakawa T, The basis for toxicity of certain cryoprotectants: a hypothesis. Cryobiology, 1990. 27: p. 401–415.
-
- Arav A, et al., Rat hindlimb cryopreservation and transplantation: A step toward “organ banking”. Am J Transplant, 2017. 17(1): p. 2820–2828. - PubMed
-
- Brunet A, et al., AKT promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell, 1999. 96: p. 857–868. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

