Postsynaptic SNARE Proteins: Role in Synaptic Transmission and Plasticity
- PMID: 30458218
- PMCID: PMC6525081
- DOI: 10.1016/j.neuroscience.2018.11.012
Postsynaptic SNARE Proteins: Role in Synaptic Transmission and Plasticity
Abstract
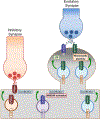
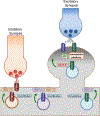
Soluble N-ethylmaleimide sensitive factor attachment protein receptor (SNARE) proteins mediate membrane fusion events in eukaryotic cells. Traditionally recognized as major players in regulating presynaptic neurotransmitter release, accumulative evidence over recent years has identified several SNARE proteins implicated in important postsynaptic processes such as neurotransmitter receptor trafficking and synaptic plasticity. Here we analyze the emerging data revealing this novel functional dimension for SNAREs with a focus on the molecular specialization of vesicular recycling and fusion in dendrites compared to those at axon terminals and its impact in synaptic transmission and plasticity.
Keywords: neurotransmitter receptor trafficking; postsynaptic SNARE proteins; postsynaptic exocytosis; synaptic plasticity.
Copyright © 2018 IBRO. Published by Elsevier Ltd. All rights reserved.
Figures

References
-
- Advani RJ, Bae HR, Bock JB, Chao DS, Doung YC, Prekeris R, Yoo JS, Scheller RH (1998), Seven novel mammalian SNARE proteins localize to distinct membrane compartments. J Biol Chem 273:10317–10324 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

