First trimester medication use in pregnancy in Cameroon: a multi-hospital survey
- PMID: 30458752
- PMCID: PMC6245902
- DOI: 10.1186/s12884-018-2081-x
First trimester medication use in pregnancy in Cameroon: a multi-hospital survey
Abstract
Background: There is a paucity of epidemiological data on medication use in pregnancy in Cameroon.
Methods: Between March and August 2015, 795 pregnant women attending 8 urban and 12 rural hospitals in Cameroon for antenatal (ANC) or other care were interviewed on first trimester medication use using structured questionnaires. Multivariate logistic regression was used to analyse the association of 18 sociodemographic factors with medication use.
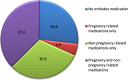
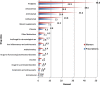
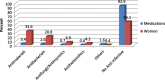
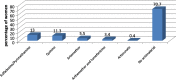
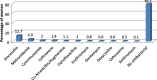
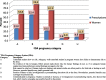
Results: A total of 582 (73.2%) women took at least one orthodox (Western) medication during the first trimester, 543 (68.3%) women a non-pregnancy related orthodox medication, and 336 (42.3%)women a pregnancy related orthodox medication. 44% of the women took anti-infectives including antimalarials (33.6%) and antibiotics (20.8%).The other most common medications were analgesics (48.8%) and antianaemias (38.6%). Sulfadoxine/pyrimethamine, contraindicated in the first trimester of pregnancy, was the most commonly used antimalarial(13% of women).0.2% of women reported antiretroviral use. Almost 80% of all orthodox medications consumed by women were purchased from the hospital. 12.8% of the women self-prescribed. Health unit and early gestational age at ANC booking were consistent determinants of prescribing of non-pregnancy related, pregnancy related and anti-infective medications. Illness and opinion on the safety of orthodox medications were determinants of the use of non-pregnancy related medications and anti-infectives. Age and parity were associated only with non-pregnancy related medications.
Conclusion: This study has confirmed the observations of studies across Africa indicating the increasing use of medications during pregnancy. This is an indication that access to medicine is improving and more emphasis now must be placed on medication safety systems targeting pregnant women, especially during the first trimester when the risk of teratogenicity is highest.
Keywords: Cameroon; Medication; determinants; drug safety; drug use; pharmacoepidemiology; pharmacovigilance; pregnancy.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval for this study was initially obtained from the Institute of Nursing and Health Research Governance Filter Committee of Ulster University and later from the National Ethics Committee in Cameroon (CNEC). All participants were given full orientation to the study and signed a consent form. Written or verbal consent from a parent or legal guardian on behalf of the participants under the age of 16 was discussed and waved by CNEC during the ethical review process.
Consent for publication
Not Applicable
Competing interests
We declare no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Adam MP, Polifka JE, Friedman JM. Evolving knowledge of the teratogenicity of medications in human pregnancy. In American Journal of Medical Genetics Part C: Seminars in Medical Genetics 2011157(3):175–182). Wiley Subscription Services, Inc., A Wiley Company. - PubMed
-
- World Health Organization. World health statistics: monitoring health for the SDGs, sustainable development goals. In World health statistics 2017: monitoring health for the SDGs, sustainable development goals; 2017.http://www.who.int/gender-equity-rights/knowledge/world-health-statistic.... Accessed 18 Sept 2017.
-
- World Health Organisation. Mental Health Atlas Available at. [WHO website].2011 http://www.who.int/mental_health/evidence/atlas/profiles/cmr_mh_profile.pdf. Accessed 18 Sept 2017.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

