Molecular Basis for the Evolution of Species-Specific Hemoglobin Capture by Staphylococcus aureus
- PMID: 30459189
- PMCID: PMC6247092
- DOI: 10.1128/mBio.01524-18
Molecular Basis for the Evolution of Species-Specific Hemoglobin Capture by Staphylococcus aureus
Abstract
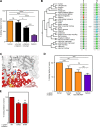
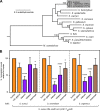
Metals are a limiting resource for pathogenic bacteria and must be scavenged from host proteins. Hemoglobin provides the most abundant source of iron in the human body and is required by several pathogens to cause invasive disease. However, the consequences of hemoglobin evolution for bacterial nutrient acquisition remain unclear. Here we show that the α- and β-globin genes exhibit strikingly parallel signatures of adaptive evolution across simian primates. Rapidly evolving sites in hemoglobin correspond to binding interfaces of IsdB, a bacterial hemoglobin receptor harbored by pathogenic Staphylococcus aureus Using an evolution-guided experimental approach, we demonstrate that the divergence between primates and staphylococcal isolates governs hemoglobin recognition and bacterial growth. The reintroduction of putative adaptive mutations in α- or β-globin proteins was sufficient to impair S. aureus binding, providing a mechanism for the evolution of disease resistance. These findings suggest that bacterial hemoprotein capture has driven repeated evolutionary conflicts with hemoglobin during primate descent.IMPORTANCE During infection, bacteria must steal metals, including iron, from the host tissue. Therefore, pathogenic bacteria have evolved metal acquisition systems to overcome the elaborate processes mammals use to withhold metal from pathogens. Staphylococcus aureus uses IsdB, a hemoglobin receptor, to thieve iron-containing heme from hemoglobin within human blood. We find evidence that primate hemoglobin has undergone rapid evolution at protein surfaces contacted by IsdB. Additionally, variation in the hemoglobin sequences among primates, or variation in IsdB of related staphylococci, reduces bacterial hemoglobin capture. Together, these data suggest that S. aureus has evolved to recognize human hemoglobin in the face of rapid evolution at the IsdB binding interface, consistent with repeated evolutionary conflicts in the battle for iron during host-pathogen interactions.
Keywords: Staphylococcus aureus; evolution; heme transport; hemoglobin; host-pathogen interactions; iron acquisition.
Copyright © 2018 Choby et al.
Figures




Similar articles
-
Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization.J Bacteriol. 2006 Dec;188(24):8421-9. doi: 10.1128/JB.01335-06. Epub 2006 Oct 13. J Bacteriol. 2006. PMID: 17041042 Free PMC article.
-
IsdB-dependent hemoglobin binding is required for acquisition of heme by Staphylococcus aureus.J Infect Dis. 2014 Jun 1;209(11):1764-72. doi: 10.1093/infdis/jit817. Epub 2013 Dec 13. J Infect Dis. 2014. PMID: 24338348 Free PMC article.
-
Specificity for human hemoglobin enhances Staphylococcus aureus infection.Cell Host Microbe. 2010 Dec 16;8(6):544-50. doi: 10.1016/j.chom.2010.11.002. Cell Host Microbe. 2010. PMID: 21147468 Free PMC article.
-
Structural biology of heme binding in the Staphylococcus aureus Isd system.J Inorg Biochem. 2010 Mar;104(3):341-8. doi: 10.1016/j.jinorgbio.2009.09.012. Epub 2009 Sep 26. J Inorg Biochem. 2010. PMID: 19853304 Review.
-
A battle for iron: host sequestration and Staphylococcus aureus acquisition.Microbes Infect. 2012 Mar;14(3):217-27. doi: 10.1016/j.micinf.2011.11.001. Epub 2011 Nov 15. Microbes Infect. 2012. PMID: 22123296 Free PMC article. Review.
Cited by
-
Staphylococcus schweitzeri-An Emerging One Health Pathogen?Microorganisms. 2022 Apr 2;10(4):770. doi: 10.3390/microorganisms10040770. Microorganisms. 2022. PMID: 35456820 Free PMC article. Review.
-
A Rapidly Evolving Polybasic Motif Modulates Bacterial Detection by Guanylate Binding Proteins.mBio. 2020 May 19;11(3):e00340-20. doi: 10.1128/mBio.00340-20. mBio. 2020. PMID: 32430466 Free PMC article.
-
Mechanisms of host adaptation by bacterial pathogens.FEMS Microbiol Rev. 2024 Jun 20;48(4):fuae019. doi: 10.1093/femsre/fuae019. FEMS Microbiol Rev. 2024. PMID: 39003250 Free PMC article. Review.
-
TLR4 sensing of IsdB of Staphylococcus aureus induces a proinflammatory cytokine response via the NLRP3-caspase-1 inflammasome cascade.mBio. 2024 Jan 16;15(1):e0022523. doi: 10.1128/mbio.00225-23. Epub 2023 Dec 19. mBio. 2024. PMID: 38112465 Free PMC article.
-
Diversification of CD1 Molecules Shapes Lipid Antigen Selectivity.Mol Biol Evol. 2021 May 19;38(6):2273-2284. doi: 10.1093/molbev/msab022. Mol Biol Evol. 2021. PMID: 33528563 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

