Antibody-Mediated Protection against Plasmodium Sporozoites Begins at the Dermal Inoculation Site
- PMID: 30459199
- PMCID: PMC6247089
- DOI: 10.1128/mBio.02194-18
Antibody-Mediated Protection against Plasmodium Sporozoites Begins at the Dermal Inoculation Site
Abstract
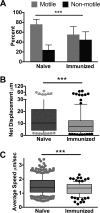
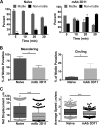
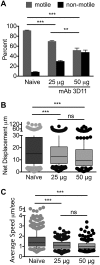
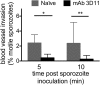
Plasmodium sporozoites are injected into the skin as mosquitoes probe for blood. From here, they migrate through the dermis to find blood vessels which they enter in order to be rapidly carried to the liver, where they invade hepatocytes and develop into the next life cycle stage, the exoerythrocytic stage. Once sporozoites enter the blood circulation, they are found in hepatocytes within minutes. In contrast, sporozoite exit from the inoculation site resembles a slow trickle and occurs over several hours. Thus, sporozoites spend the majority of their extracellular time at the inoculation site, raising the hypothesis that this is when the malarial parasite is most vulnerable to antibody-mediated destruction. Here, we investigate this hypothesis and demonstrate that the neutralizing capacity of circulating antibodies is greater at the inoculation site than in the blood circulation. Furthermore, these antibodies are working, at least in part, by impacting sporozoite motility at the inoculation site. Using actively and passively immunized mice, we found that most parasites are either immobilized at the site of injection or display reduced motility, particularly in their net displacement. We also found that antibodies severely impair the entry of sporozoites into the bloodstream. Overall, our data suggest that antibodies targeting the migratory sporozoite exert a large proportion of their protective effect at the inoculation site.IMPORTANCE Studies in experimental animal models and humans have shown that antibodies against Plasmodium sporozoites abolish parasite infectivity and provide sterile immunity. While it is well documented that these antibodies can be induced after immunization with attenuated parasites or subunit vaccines, the mechanisms by and location in which they neutralize parasites have not been fully elucidated. Here, we report studies indicating that these antibodies display a significant portion of their protective effect in the skin after injection of sporozoites and that one mechanism by which they work is by impairing sporozoite motility, thus diminishing their ability to reach blood vessels. These results suggest that immune protection against malaria begins at the earliest stages of parasite infection and emphasize the need of performing parasite challenge in the skin for the evaluation of protective immunity.
Keywords: antibodies; malaria; preerythrocytic; skin; sporozoites; vaccine.
Copyright © 2018 Flores-Garcia et al.
Figures



Comment in
-
Shedding Light on the Role of the Skin in Vaccine-Induced Protection against the Malaria Sporozoite.mBio. 2018 Dec 11;9(6):e02555-18. doi: 10.1128/mBio.02555-18. mBio. 2018. PMID: 30538191 Free PMC article.
-
Further Mechanisms and Locations in Which Antisporozoite Antibodies Neutralize Malaria Sporozoites.mBio. 2019 Sep 10;10(5):e01588-19. doi: 10.1128/mBio.01588-19. mBio. 2019. PMID: 31506308 Free PMC article. No abstract available.
Similar articles
-
Longitudinal analysis of Plasmodium sporozoite motility in the dermis reveals component of blood vessel recognition.Elife. 2015 Aug 13;4:e07789. doi: 10.7554/eLife.07789. Elife. 2015. PMID: 26271010 Free PMC article.
-
Extrahepatic exoerythrocytic forms of rodent malaria parasites at the site of inoculation: clearance after immunization, susceptibility to primaquine, and contribution to blood-stage infection.Infect Immun. 2012 Jun;80(6):2158-64. doi: 10.1128/IAI.00246-12. Epub 2012 Mar 19. Infect Immun. 2012. PMID: 22431651 Free PMC article.
-
Intravital microscopy demonstrating antibody-mediated immobilisation of Plasmodium berghei sporozoites injected into skin by mosquitoes.Int J Parasitol. 2004 Aug;34(9):991-6. doi: 10.1016/j.ijpara.2004.05.005. Int J Parasitol. 2004. PMID: 15313126
-
Imaging mosquito transmission of Plasmodium sporozoites into the mammalian host: immunological implications.Parasitol Int. 2014 Feb;63(1):150-64. doi: 10.1016/j.parint.2013.09.010. Epub 2013 Sep 21. Parasitol Int. 2014. PMID: 24060541 Review.
-
Plasmodium sporozoite-host interactions from the dermis to the hepatocyte.Curr Opin Microbiol. 2009 Aug;12(4):401-7. doi: 10.1016/j.mib.2009.06.006. Epub 2009 Jul 14. Curr Opin Microbiol. 2009. PMID: 19608456 Free PMC article. Review.
Cited by
-
Plasmodium sporozoites induce regulatory macrophages.PLoS Pathog. 2020 Sep 8;16(9):e1008799. doi: 10.1371/journal.ppat.1008799. eCollection 2020 Sep. PLoS Pathog. 2020. PMID: 32898164 Free PMC article.
-
Chemoprophylaxis vaccination with a Plasmodium liver stage autophagy mutant affords enhanced and long-lasting protection.NPJ Vaccines. 2021 Aug 10;6(1):98. doi: 10.1038/s41541-021-00360-1. NPJ Vaccines. 2021. PMID: 34376691 Free PMC article.
-
Coimmunization with Preerythrocytic Antigens alongside Circumsporozoite Protein Can Enhance Sterile Protection against Plasmodium Sporozoite Infection.Microbiol Spectr. 2023 Feb 27;11(2):e0379122. doi: 10.1128/spectrum.03791-22. Online ahead of print. Microbiol Spectr. 2023. PMID: 36847573 Free PMC article.
-
Shedding Light on the Role of the Skin in Vaccine-Induced Protection against the Malaria Sporozoite.mBio. 2018 Dec 11;9(6):e02555-18. doi: 10.1128/mBio.02555-18. mBio. 2018. PMID: 30538191 Free PMC article.
-
The P. falciparum CSP repeat region contains three distinct epitopes required for protection by antibodies in vivo.PLoS Pathog. 2021 Nov 8;17(11):e1010042. doi: 10.1371/journal.ppat.1010042. eCollection 2021 Nov. PLoS Pathog. 2021. PMID: 34748617 Free PMC article.
References
-
- White MT, Verity R, Griffin JT, Asante KP, Owusu-Agyei S, Greenwood B, Drakeley C, Gesase S, Lusingu J, Ansong D, Adjei S, Agbenyega T, Ogutu B, Otieno L, Otieno W, Agnandji ST, Lell B, Kremsner P, Hoffman I, Martinson F, Kamthunzu P, Tinto H, Valea I, Sorgho H, Oneko M, Otieno K, Hamel MJ, Salim N, Mtoro A, Abdulla S, Aide P, Sacarlal J, Aponte JJ, Njuguna P, Marsh K, Bejon P, Riley EM, Ghani AC. 2015. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis 15:1450–1458. doi:10.1016/S1473-3099(15)00239-X. - DOI - PMC - PubMed
-
- White MT, Bejon P, Olotu A, Griffin JT, Riley EM, Kester KE, Ockenhouse CF, Ghani AC. 2013. The relationship between RTS,S vaccine-induced antibodies, CD4+ T cell responses and protection against Plasmodium falciparum infection. PLoS One 8:e61395. doi:10.1371/journal.pone.0061395. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
