Absence of functional compensation between coagulation factor VIII and plasminogen in double-knockout mice
- PMID: 30459211
- PMCID: PMC6258924
- DOI: 10.1182/bloodadvances.2018024851
Absence of functional compensation between coagulation factor VIII and plasminogen in double-knockout mice
Abstract
Plasminogen deficiency is associated with severely compromised fibrinolysis and extravascular deposition of fibrin. In contrast, coagulation factor VIII (FVIII) deficiency leads to prolonged and excessive bleeding. Based on opposing biological functions of plasminogen and FVIII deficiencies, we hypothesized that genetic elimination of FVIII would alleviate the systemic formation of fibrin deposits associated with plasminogen deficiency and, in turn, elimination of plasminogen would limit bleeding symptoms associated with FVIII deficiency. Mice with single and combined deficiencies of FVIII (F8-/-) and plasminogen (Plg-/-) were evaluated for phenotypic characteristics of plasminogen deficiency, including wasting disease, shortened lifespan, rectal prolapse, and multiorgan fibrin deposition. Conversely, to specifically examine the role of plasmin-mediated fibrinolysis on bleeding caused by FVIII deficiency, F8-/- and F8-/-/Plg-/- mice were subjected to a bleeding challenge. Mice with a combined deficiency in FVIII and plasminogen displayed no phenotypic differences relative to mice with single FVIII or plasminogen deficiency. Plg-/- and F8-/-/Plg-/- mice exhibited the same penetrance and severity of wasting disease, rectal prolapse, extravascular fibrin deposits, and reduced viability. Furthermore, following a tail vein-bleeding challenge, no significant differences in bleeding times or total blood loss could be detected between F8-/- and F8-/-/Plg-/- mice. Moreover, F8-/- and F8-/-/Plg-/- mice responded similarly to recombinant FVIII (rFVIII) therapy. In summary, the pathological phenotype of Plg-/- mice developed independently of FVIII-dependent coagulation, and elimination of plasmin-driven fibrinolysis did not play a significant role in a nonmucosal bleeding model in hemophilia A mice.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: R.S., C.D.L., K.A., and T.K. are or were employees of Novo Nordisk A/S during the study. C.D.L., K.A., and T.K. are minor shareholders of Novo Nordisk A/S. At the University of Copenhagen, L.H.O. was affiliated with the in vivo pharmacology research center LIFEPHARM, which was supported by Novo Nordisk A/S. M.J.F. has previously received research funding from Novo Nordisk A/S, but in a research area separate and distinct from the plasminogen and hemophilia A studies reported in the present manuscript.
Figures


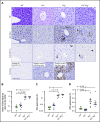
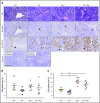

References
-
- Carmeliet P, Schoonjans L, Kieckens L, et al. . Physiological consequences of loss of plasminogen activator gene function in mice. Nature. 1994;368(6470):419-424. - PubMed
-
- Schuster V, Hügle B, Tefs K. Plasminogen deficiency. J Thromb Haemost. 2007;5(12):2315-2322. - PubMed
-
- Tefs K, Gueorguieva M, Klammt J, et al. . Molecular and clinical spectrum of type I plasminogen deficiency: a series of 50 patients. Blood. 2006;108(9):3021-3026. - PubMed
-
- Mehta R, Shapiro AD. Plasminogen deficiency. Haemophilia. 2008;14(6):1261-1268. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

