Molecular structure of the ATP-bound, phosphorylated human CFTR
- PMID: 30459277
- PMCID: PMC6294961
- DOI: 10.1073/pnas.1815287115
Molecular structure of the ATP-bound, phosphorylated human CFTR
Abstract
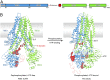
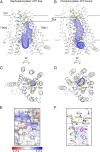
The cystic fibrosis transmembrane conductance regulator (CFTR) is an anion channel important in maintaining proper functions of the lung, pancreas, and intestine. The activity of CFTR is regulated by ATP and protein kinase A-dependent phosphorylation. To understand the conformational changes elicited by phosphorylation and ATP binding, we present here the structure of phosphorylated, ATP-bound human CFTR, determined by cryoelectron microscopy to 3.2-Å resolution. This structure reveals the position of the R domain after phosphorylation. By comparing the structures of human CFTR and zebrafish CFTR determined under the same condition, we identified common features essential to channel gating. The differences in their structures indicate plasticity permitted in evolution to achieve the same function. Finally, the structure of CFTR provides a better understanding of why the G178R, R352Q, L927P, and G970R/D mutations would impede conformational changes of CFTR and lead to cystic fibrosis.
Keywords: ABC transporter; anion channel; cryo-EM; human CFTR.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Boat TF, Welsh MJ, Beaudet AL. In: The Metabolic Basis of Inherited Disease. 6th Ed. Scriver CR, Beaudet Al, Sly WS, Valle D, editors. McGraw-Hill; New York: 1989. pp. 2649–2680.
-
- Rommens JM, et al. Identification of the cystic fibrosis gene: Chromosome walking and jumping. Science. 1989;245:1059–1065. - PubMed
-
- Elborn JS. Cystic fibrosis. Lancet. 2016;388:2519–2531. - PubMed
-
- Cheng SH, et al. Phosphorylation of the R domain by cAMP-dependent protein kinase regulates the CFTR chloride channel. Cell. 1991;66:1027–1036. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

