Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have differential effects on cystic fibrosis macrophage function
- PMID: 30459435
- PMCID: PMC6244248
- DOI: 10.1038/s41598-018-35151-7
Cystic fibrosis transmembrane conductance regulator (CFTR) modulators have differential effects on cystic fibrosis macrophage function
Abstract
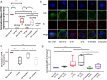
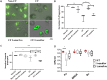
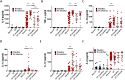
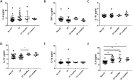
Despite the addition of cystic fibrosis transmembrane conductance regulator (CFTR) modulators to the cystic fibrosis (CF) treatment regimen, patients with CF continue to suffer from chronic bacterial infections that lead to progressive respiratory morbidity. Host immunity, and macrophage dysfunction specifically, has an integral role in the inability of patients with CF to clear bacterial infections. We sought to characterize macrophage responses to CFTR modulator treatment as we hypothesized that there would be differential effects based on patient genotype. Human CF and non-CF peripheral blood monocyte-derived macrophages (MDMs) were analyzed for CFTR expression, apoptosis, polarization, phagocytosis, bacterial killing, and cytokine production via microscopy, flow cytometry, and ELISA-based assays. Compared to non-CF MDMs, CF MDMs display decreased CFTR expression, increased apoptosis, and decreased phagocytosis. CFTR expression increased and apoptosis decreased in response to ivacaftor or lumacaftor/ivacaftor therapy, and phagocytosis improved with ivacaftor alone. Ivacaftor restored CF macrophage polarization responses to non-CF levels and reduced Pseudomonas aeruginosa bacterial burden, but did not reduce other bacterial loads. Macrophage inflammatory cytokine production decreased in response to ivacaftor alone. In summary, ivacaftor and lumacaftor/ivacaftor have differential impacts on macrophage function with minimal changes observed in CF patients treated with lumacaftor/ivacaftor. Overall improvements in macrophage function in ivacaftor-treated CF patients result in modestly improved macrophage-mediated bacterial killing.
Conflict of interest statement
B.T.K. is a member of the US CF Advisory Board for Vertex Pharmaceuticals to provide advice on clinical trial development.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

