Understanding the Thalidomide Chirality in Biological Processes by the Self-disproportionation of Enantiomers
- PMID: 30459439
- PMCID: PMC6244226
- DOI: 10.1038/s41598-018-35457-6
Understanding the Thalidomide Chirality in Biological Processes by the Self-disproportionation of Enantiomers
Abstract
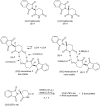
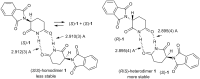
Twenty years after the thalidomide disaster in the late 1950s, Blaschke et al. reported that only the (S)-enantiomer of thalidomide is teratogenic. However, other work has shown that the enantiomers of thalidomide interconvert in vivo, which begs the question: why is teratogen activity not observed in animal experiments that use (R)-thalidomide given the ready in vivo racemization ("thalidomide paradox")? Herein, we disclose a hypothesis to explain this "thalidomide paradox" through the in-vivo self-disproportionation of enantiomers. Upon stirring a 20% ee solution of thalidomide in a given solvent, significant enantiomeric enrichment of up to 98% ee was observed reproducibly in solution. We hypothesize that a fraction of thalidomide enantiomers epimerizes in vivo, followed by precipitation of racemic thalidomide in (R/S)-heterodimeric form. Thus, racemic thalidomide is most likely removed from biological processes upon racemic precipitation in (R/S)-heterodimeric form. On the other hand, enantiomerically pure thalidomide remains in solution, affording the observed biological experimental results: the (S)-enantiomer is teratogenic, while the (R)-enantiomer is not.
Conflict of interest statement
The authors declare no competing interests.
Figures
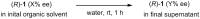

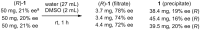

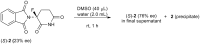
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

