IL-23R Signaling Plays No Role in Myocardial Infarction
- PMID: 30459442
- PMCID: PMC6244091
- DOI: 10.1038/s41598-018-35188-8
IL-23R Signaling Plays No Role in Myocardial Infarction
Abstract
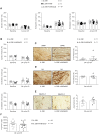
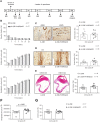
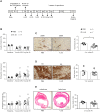
Ischemic heart diseases are the most frequent diseases in the western world. Apart from Interleukin (IL-)1, inflammatory therapeutic targets in the clinic are still missing. Interestingly, opposing roles of the pro-inflammatory cytokine IL-23 have been described in cardiac ischemia in mice. IL-23 is a composite cytokine consisting of p19 and p40 which binds to IL-23R and IL-12Rβ1 to initiate signal transduction characterized by activation of the Jak/STAT, PI3K and Ras/Raf/MAPK pathways. Here, we generate IL-23R-Y416FΔICD signaling deficient mice and challenged these mice in close- and open-chest left anterior descending coronary arteria ischemia/reperfusion experiments. Our experiments showed only minimal changes in all assayed parameters in IL-23R signaling deficient mice compared to wild-type mice in ischemia and for up to four weeks of reperfusion, including ejection fraction, endsystolic volume, enddiastolic volume, infarct size, gene regulation and α smooth muscle actin (αSMA) and Hyaluronic acid (HA) protein expression. Moreover, injection of IL-23 in wild-type mice after LAD ischemia/reperfusion had also no influence on the outcome of the healing phase. Our data showed that IL-23R deficiency has no effects in myocardial I/R.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Savvatis K, et al. Interleukin-23 deficiency leads to impaired wound healing and adverse prognosis after myocardial infarction. Circulation: Heart Failure. 2014;7:161–171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

