Human Adipose Tissue-Derived Mesenchymal Stem Cells Attenuate Atopic Dermatitis by Regulating the Expression of MIP-2, miR-122a-SOCS1 Axis, and Th1/Th2 Responses
- PMID: 30459600
- PMCID: PMC6232252
- DOI: 10.3389/fphar.2018.01175
Human Adipose Tissue-Derived Mesenchymal Stem Cells Attenuate Atopic Dermatitis by Regulating the Expression of MIP-2, miR-122a-SOCS1 Axis, and Th1/Th2 Responses
Abstract
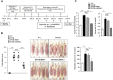
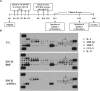
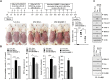
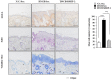
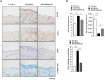
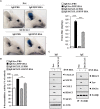
The objective of this study was to investigate the effect of human adipose tissue-derived mesenchymal stem cells (AdMSCs) on atopic dermatitis (AD) in the BALB/c mouse model. The AdMSCs attenuated clinical symptoms associated with AD, decreased numbers of degranulated mast cells (MCs), IgE level, amount of histamine released, and prostaglandin E2 level. Atopic dermatitis increased the expression levels of cytokines/chemokines, such as interleukin-5 (IL-5), macrophage inflammatory protein-1ß (MIP-1ß), MIP-2, chemokine (C-C motif) ligand 5 (CCL5), and IL-17, in BALB/c mouse. The AdMSCs showed decreased expression levels of these cytokines in the mouse model of AD. In vivo downregulation of MIP-2 attenuated the clinical symptoms associated with AD. Atopic dermatitis increased the expression levels of hallmarks of allergic inflammation, induced interactions of Fc𝜀RIβ with histone deacetylase 3 (HDAC3) and Lyn, increased ß-hexosaminidase activity, increased serum IgE level, and increased the amount of histamine released in an MIP-2-dependent manner. Downregulation of MIP-2 increased the levels of several miRNAs, including miR-122a-5p. Mouse miR-122a-5p mimic inhibited AD, while suppressor of cytokine signaling 1 (SOCS1), a predicted downstream target of miR-122a-5p, was required for AD. The downregulation of SOCS1 decreased the expression levels of MIP-2 and chemokine (C-X-C motif) ligand 13 (CXCL13) in the mouse model of AD. The downregulation of CXCL13 attenuated AD and allergic inflammation such as passive cutaneous anaphylaxis. The role of T cell transcription factors in AD was also investigated. Atopic dermatitis increased the expression levels of T-bet and GATA-3 [transcription factors of T-helper 1 (Th1) and T-helper 2 (Th2) cells, respectively] but decreased the expression of Foxp3, a transcription factor of regulatory T (Treg) cells, in an SOCS1-dependent manner. In addition to this, miR-122a-5p mimic also prevented AD from regulating the expression of T-bet, GATA-3, and Foxp3. Atopic dermatitis increased the expression of cluster of differentiation 163 (CD163), a marker of M2 macrophages, but decreased the expression of inducible nitric oxide synthase (iNOS), a marker of M1 macrophages. Additionally, SOCS1 and miR-122a-5p mimic regulated the expression of CD163 and iNOS in the mouse model of AD. Experiments employing conditioned medium showed interactions between MCs and macrophages in AD. The conditioned medium of AdMSCs, but not the conditioned medium of human dermal fibroblasts, negatively inhibited the features of allergic inflammation. In summary, we investigated the anti-atopic effects of AdMSCs, identified targets of AdMSCs, and determined the underlying mechanism for the anti-atopic effects of AdMSCs.
Keywords: MIP-2; SOCS1; adipose tissue-derived stem cells; atopic dermatitis; cellular interactions; miR-122a-5p; targets of stem cells.
Figures









Similar articles
-
HDAC6 and CXCL13 Mediate Atopic Dermatitis by Regulating Cellular Interactions and Expression Levels of miR-9 and SIRT1.Front Pharmacol. 2021 Sep 13;12:691279. doi: 10.3389/fphar.2021.691279. eCollection 2021. Front Pharmacol. 2021. PMID: 34588978 Free PMC article.
-
Homoharringtonine Inhibits Allergic Inflammations by Regulating NF-κB-miR-183-5p-BTG1 Axis.Front Pharmacol. 2020 Jul 7;11:1032. doi: 10.3389/fphar.2020.01032. eCollection 2020. Front Pharmacol. 2020. PMID: 32733254 Free PMC article.
-
Effect of microfragmented adipose tissue on osteoarthritic synovial macrophage factors.J Cell Physiol. 2019 Apr;234(4):5044-5055. doi: 10.1002/jcp.27307. Epub 2018 Sep 6. J Cell Physiol. 2019. PMID: 30187478
-
Regulation of the MIR155 host gene in physiological and pathological processes.Gene. 2013 Dec 10;532(1):1-12. doi: 10.1016/j.gene.2012.12.009. Epub 2012 Dec 14. Gene. 2013. PMID: 23246696 Review.
-
The role of cytokines/chemokines in the pathogenesis of atopic dermatitis.Curr Probl Dermatol. 2011;41:80-92. doi: 10.1159/000323299. Epub 2011 May 12. Curr Probl Dermatol. 2011. PMID: 21576949 Review.
Cited by
-
Therapeutic effects of mesenchymal stem cells and their derivatives in common skin inflammatory diseases: Atopic dermatitis and psoriasis.Front Immunol. 2023 Feb 20;14:1092668. doi: 10.3389/fimmu.2023.1092668. eCollection 2023. Front Immunol. 2023. PMID: 36891306 Free PMC article. Review.
-
Schizonepeta tenuifolia Briq-Saposhnikovia divaricata decoction alleviates atopic dermatitis via downregulating macrophage TRPV1.Front Pharmacol. 2024 Aug 27;15:1413513. doi: 10.3389/fphar.2024.1413513. eCollection 2024. Front Pharmacol. 2024. PMID: 39257398 Free PMC article.
-
Adipose-derived stem cells in immune-related skin disease: a review of current research and underlying mechanisms.Stem Cell Res Ther. 2024 Feb 8;15(1):37. doi: 10.1186/s13287-023-03561-8. Stem Cell Res Ther. 2024. PMID: 38331803 Free PMC article. Review.
-
Noval advance of histone modification in inflammatory skin diseases and related treatment methods.Front Immunol. 2024 Jan 3;14:1286776. doi: 10.3389/fimmu.2023.1286776. eCollection 2023. Front Immunol. 2024. PMID: 38235133 Free PMC article. Review.
-
Stem Cells from Human Exfoliated Deciduous Teeth Attenuate Atopic Dermatitis Symptoms in Mice through Modulating Immune Balance and Skin Barrier Function.Mediators Inflamm. 2022 Jul 21;2022:6206883. doi: 10.1155/2022/6206883. eCollection 2022. Mediators Inflamm. 2022. PMID: 35909660 Free PMC article.
References
-
- Chimenti I., Smith R. R., Li T. S., Gerstenblith G., Messina E., Giacomello A., et al. (2010). Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ. Res. 106 971–980. 10.1161/CIRCRESAHA.109.210682 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

