Lactobacillus crispatus BC5 Interferes With Chlamydia trachomatis Infectivity Through Integrin Modulation in Cervical Cells
- PMID: 30459737
- PMCID: PMC6232233
- DOI: 10.3389/fmicb.2018.02630
Lactobacillus crispatus BC5 Interferes With Chlamydia trachomatis Infectivity Through Integrin Modulation in Cervical Cells
Abstract
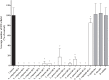
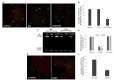
Lactobacilli play a crucial role in maintaining the ecological equilibrium of the vaginal niche, preventing the colonization of exogenous microorganisms. Although many studies have discussed the mechanisms displayed by lactobacilli in counteracting several urogenital pathogens, a few data are available on the interaction between lactobacilli and Chlamydia trachomatis. This study aimed to elucidate the molecular bases of the interaction among vaginal lactobacilli, the sexually transmitted pathogen C. trachomatis and the epithelial cervical cells. We evaluated the in vitro activity of 15 Lactobacillus strains, belonging to different species (i.e., L. crispatus, L. gasseri, L. vaginalis), against C. trachomatis. In particular, we evaluated the capability of lactobacilli cells to interfere with C. trachomatis infection in HeLa cells, by exclusion assays. Lactobacilli significantly reduced C. trachomatis infectivity, being L. crispatus the most active species. Although a dose-dependent effect was noticed, a significant antagonistic activity was maintained even at lower doses. As other Gram-positive bacteria (i.e., Streptococcus agalactiae, Enterococcus faecalis, and Bacillus subtilis) failed to interfere with C. trachomatis infectivity, Lactobacillus activity proved to be specific. The potential mechanism of protection was investigated in Lactobacillus crispatus BC5, chosen as the model strain. The incubation of HeLa cell line with BC5 cells induced important modifications in the epithelial plasma membrane, by altering lipid composition and α5 integrin subunit exposure. When α5 integrin subunits were masked by a specific blocking antibody or ITGA5 gene expression was silenced, Chlamydia infection was significantly reduced. It follows that α5 integrin subunit is crucial for the pathogen infection process, and the anti-Chlamydia activity can be directly linked to membrane properties modifications in cervical cells. The three Gram-positive bacteria used as controls failed to modify the expression of α5β1 integrin. In conclusion, we identified a potential molecular mechanism at the basis of the protection exerted by L. crispatus BC5 against C. trachomatis, getting insights into the role of the cervico-vaginal microbiota for the woman's health.
Keywords: Chlamydia trachomatis; HeLa cells; STIs; integrin; lactobacilli; probiotics; women health.
Figures
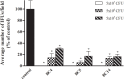

References
-
- Alcaide M. L., Chisembele M., Malupande E., Arheart K., Fischl M., Jones D. L. (2015). A cross-sectional study of bacterial vaginosis, intravaginal practices and HIV genital shedding; implications for HIV transmission and women’s health. BMJ Open 5:e009036. 10.1136/bmjopen-2015-009036 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

