Suppression of Fluconazole Resistant Candida albicans Biofilm Formation and Filamentation by Methylindole Derivatives
- PMID: 30459738
- PMCID: PMC6232606
- DOI: 10.3389/fmicb.2018.02641
Suppression of Fluconazole Resistant Candida albicans Biofilm Formation and Filamentation by Methylindole Derivatives
Abstract
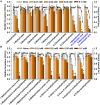
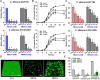
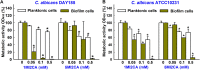
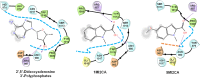
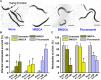
Candida albicans is an opportunistic fungal pathogen and most prevalent species among clinical outbreaks. It causes a range of infections, including from mild mucosal infections to serious life-threatening candidemia and disseminated candidiasis. Multiple virulence factors account for the pathogenic nature of C. albicans, and its morphological transition from budding yeast to hyphal form and subsequent biofilm formation is regarded as the most important reason for the severity of Candida infections. To address the demanding need for novel antifungals, we investigated the anti-biofilm activities of various methylindoles against C. albicans using a crystal violet assay, and the metabolic activity was assessed by using a 2,3-bis (2-methoxy-4-nitro-5-sulfo-phenyl)-2H-tetrazolium-5-carboxanilide reduction assay. Changes in biofilm morphologies and thicknesses were determined by confocal laser scanning microscopy and scanning electron microscopy, respectively. Of the 21 methylindoles tested, 1-methylindole-2-carboxylic acid (1MI2CA) at 0.1 mM (17.5 μg ml-1) and 5-methylindole-2-carboxylic acid (5MI2CA) at 0.1 mM effectively inhibited biofilm formation by C. albicans DAY185 and ATCC10231 strains. Moreover, 1MI2CA and 5MI2CA both effectively inhibited hyphal formation, and thus, improved C. albicans infected nematode survival without inducing acute toxic effects. Furthermore, our in silico molecular modeling findings were in-line with in vitro observations. This study provides information useful for the development of novel strategies targeting candidiasis and biofilm-related infections.
Keywords: C. albicans; C. elegans; biofilm; filamentation; methylindoles.
Figures

References
-
- CDC (2013). Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. Available at: https://www.cdc.gov/drugresistance/biggest_threats.html
-
- Desai N. C., Patel B. Y., Dave B. P. (2017). Synthesis and antimicrobial activity of novel quinoline derivatives bearing pyrazoline and pyridine analogues. Med. Chem. Res. 26 109–119. 10.1007/s00044-016-1732-6 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

