The Pentraxins 1975-2018: Serendipity, Diagnostics and Drugs
- PMID: 30459761
- PMCID: PMC6232782
- DOI: 10.3389/fimmu.2018.02382
The Pentraxins 1975-2018: Serendipity, Diagnostics and Drugs
Abstract
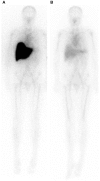
The phylogenetically ancient, pentraxin family of plasma proteins, comprises C-reactive protein (CRP) and serum amyloid P component (SAP) in humans and the homologous proteins in other species. They are composed of five, identical, non-covalently associated protomers arranged with cyclic pentameric symmetry in a disc-like configuration. Each protomer has a calcium dependent site that mediates the particular specific ligand binding responsible for all the rigorously established functional properties of these proteins. No genetic deficiency of either human CRP or SAP has been reported, nor even any sequence polymorphism in the proteins themselves. Although their actual functions in humans are therefore unknown, gene deletion studies in mice demonstrate that both proteins can contribute to innate immunity. CRP is the classical human acute phase protein, routinely measured in clinical practice worldwide to monitor disease activity. Human SAP, which is not an acute phase protein, is a universal constituent of all human amyloid deposits as a result of its avid specific binding to amyloid fibrils of all types. SAP thereby contributes to amyloid formation and persistence in vivo. Whole body radiolabelled SAP scintigraphy safely and non-invasively localizes and quantifies systemic amyloid deposits, and has transformed understanding of the natural history of amyloidosis and its response to treatment. Human SAP is also a therapeutic target, both in amyloidosis and Alzheimer's disease. Our drug, miridesap, depletes SAP from the blood and the brain and is currently being tested in the DESPIAD clinical trial in Alzheimer's disease. Meanwhile, the obligate therapeutic partnership of miridesap, to deplete circulating SAP, and dezamizumab, a humanized monoclonal anti-SAP antibody that targets residual SAP in amyloid deposits, produces unprecedented removal of amyloid from the tissues and improves organ function. Human CRP binds to dead and damaged cells in vivo and activates complement and this can exacerbate pre-existing tissue damage. The adverse effects of CRP are completely abrogated by compounds that block its binding to autologous ligands and we are developing CRP inhibitor drugs. The present personal and critical perspective on the pentraxins reports, for the first time, the key role of serendipity in our work since 1975. (345 words).
Keywords: C-reactive protein; amyloidosis; complement; dezamizumab; drugs; miridesap; pentraxin; serum amyloid P component.
Figures

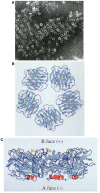
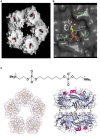

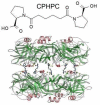
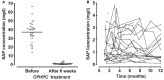

References
-
- Pepys MB. Role of complement in the induction of immunological responses. Transplant Rev. (1976) 32:93–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

