Latitude, Vitamin D, Melatonin, and Gut Microbiota Act in Concert to Initiate Multiple Sclerosis: A New Mechanistic Pathway
- PMID: 30459766
- PMCID: PMC6232868
- DOI: 10.3389/fimmu.2018.02484
Latitude, Vitamin D, Melatonin, and Gut Microbiota Act in Concert to Initiate Multiple Sclerosis: A New Mechanistic Pathway
Abstract
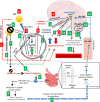
Multiple sclerosis (MS) is an inflammatory demyelinating disease of the central nervous system (CNS). While the etiology of MS is still largely unknown, scientists believe that the interaction of several endogenous and exogenous factors may be involved in this disease. Epidemiologists have seen an increased prevalence of MS in countries at high latitudes, where the sunlight is limited and where the populations have vitamin D deficiency and high melatonin levels. Although the functions and synthesis of vitamin D and melatonin are contrary to each other, both are involved in the immune system. While melatonin synthesis is affected by light, vitamin D deficiency may be involved in melatonin secretion. On the other hand, vitamin D deficiency reduces intestinal calcium absorption leading to gut stasis and subsequently increasing gut permeability. The latter allows gut microbiota to transfer more endotoxins such as lipopolysaccharides (LPS) into the blood. LPS stimulates the production of inflammatory cytokines within the CNS, especially the pineal gland. This review summarizes the current findings on the correlation between latitude, sunlight and vitamin D, and details their effects on intestinal calcium absorption, gut microbiota and neuroinflammatory mediators in MS. We also propose a new mechanistic pathway for the initiation of MS.
Keywords: gut microbiota; latitude; melatonin; multiple sclerosis; sunlight; vitamin D.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

