Pectin Methylesterases: Cell Wall Remodeling Proteins Are Required for Plant Response to Heat Stress
- PMID: 30459794
- PMCID: PMC6232315
- DOI: 10.3389/fpls.2018.01612
Pectin Methylesterases: Cell Wall Remodeling Proteins Are Required for Plant Response to Heat Stress
Abstract
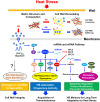
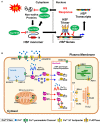
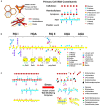
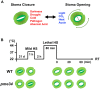
Heat stress (HS) is expected to be of increasing worldwide concern in the near future, especially with regard to crop yield and quality as a consequence of rising or varying temperatures as a result of global climate change. HS response (HSR) is a highly conserved mechanism among different organisms but shows remarkable complexity and unique features in plants. The transcriptional regulation of HSR is controlled by HS transcription factors (HSFs) which allow the activation of HS-responsive genes, among which HS proteins (HSPs) are best characterized. Cell wall remodeling constitutes an important component of plant responses to HS to maintain overall function and growth; however, little is known about the connection between cell wall remodeling and HSR. Pectin controls cell wall porosity and has been shown to exhibit structural variation during plant growth and in response to HS. Pectin methylesterases (PMEs) are present in multigene families and encode isoforms with different action patterns by removal of methyl esters to influencing the properties of cell wall. We aimed to elucidate how plant cell walls respond to certain environmental cues through cell wall-modifying proteins in connection with modifications in cell wall machinery. An overview of recent findings shed light on PMEs contribute to a change in cell-wall composition/structure. The fine-scale modulation of apoplastic calcium ions (Ca2+) content could be mediated by PMEs in response to abiotic stress for both the assembly and disassembly of the pectic network. In particular, this modulation is prevalent in guard cell walls for regulating cell wall plasticity as well as stromal aperture size, which comprise critical determinants of plant adaptation to HS. These insights provide a foundation for further research to reveal details of the cell wall machinery and stress-responsive factors to provide targets and strategies to facilitate plant adaptation.
Keywords: cell wall remodeling; guard cell wall; heat stress response; pectin; pectin methylesterase.
Figures

Similar articles
-
PECTIN METHYLESTERASE34 Contributes to Heat Tolerance through Its Role in Promoting Stomatal Movement.Plant Physiol. 2017 Jun;174(2):748-763. doi: 10.1104/pp.17.00335. Epub 2017 Apr 5. Plant Physiol. 2017. PMID: 28381503 Free PMC article.
-
Analyzing the regulatory role of heat shock transcription factors in plant heat stress tolerance: a brief appraisal.Mol Biol Rep. 2022 Jun;49(6):5771-5785. doi: 10.1007/s11033-022-07190-x. Epub 2022 Feb 19. Mol Biol Rep. 2022. PMID: 35182323 Review.
-
The Multifaceted Role of Pectin Methylesterase Inhibitors (PMEIs).Int J Mol Sci. 2018 Sep 21;19(10):2878. doi: 10.3390/ijms19102878. Int J Mol Sci. 2018. PMID: 30248977 Free PMC article. Review.
-
Genome-wide identification, phylogeny, and expression analysis of pectin methylesterases reveal their major role in cotton fiber development.BMC Genomics. 2016 Dec 7;17(1):1000. doi: 10.1186/s12864-016-3365-z. BMC Genomics. 2016. PMID: 27927181 Free PMC article.
-
Modulation of the degree and pattern of methyl-esterification of pectic homogalacturonan in plant cell walls. Implications for pectin methyl esterase action, matrix properties, and cell adhesion.J Biol Chem. 2001 Jun 1;276(22):19404-13. doi: 10.1074/jbc.M011242200. Epub 2001 Mar 6. J Biol Chem. 2001. PMID: 11278866
Cited by
-
Regulation of Proline Accumulation and Protein Secretion in Sorghum under Combined Osmotic and Heat Stress.Plants (Basel). 2024 Jul 6;13(13):1874. doi: 10.3390/plants13131874. Plants (Basel). 2024. PMID: 38999714 Free PMC article.
-
Comparative heat stress responses of three hot pepper (Capsicum annuum L.) genotypes differing temperature sensitivity.Sci Rep. 2023 Aug 30;13(1):14203. doi: 10.1038/s41598-023-41418-5. Sci Rep. 2023. PMID: 37648718 Free PMC article.
-
Alternative Oxidase Inhibition Impairs Tobacco Root Development and Root Hair Formation.Front Plant Sci. 2021 Jun 24;12:664792. doi: 10.3389/fpls.2021.664792. eCollection 2021. Front Plant Sci. 2021. PMID: 34249036 Free PMC article.
-
CCaP1/CCaP2/CCaP3 interact with plasma membrane H+-ATPases and promote thermo-responsive growth by regulating cell wall modification in Arabidopsis.Plant Commun. 2024 Jul 8;5(7):100880. doi: 10.1016/j.xplc.2024.100880. Epub 2024 Mar 14. Plant Commun. 2024. PMID: 38486455 Free PMC article.
-
Physiological and Gene Expression Changes of Clematis crassifolia and Clematis cadmia in Response to Heat Stress.Front Plant Sci. 2021 Mar 26;12:624875. doi: 10.3389/fpls.2021.624875. eCollection 2021. Front Plant Sci. 2021. PMID: 33841457 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

