Distinct regulations of ARF1 by two Aplysia Sec7 isoforms
- PMID: 30460046
- PMCID: PMC6138325
- DOI: 10.1080/19768354.2016.1276025
Distinct regulations of ARF1 by two Aplysia Sec7 isoforms
Abstract
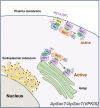
Sec7 protein is a guanine nucleotide exchange factor in the ADP-ribosylation factor (ARF) family of small GTP-binding proteins. Aplysia Sec7 proteins (ApSec7s) play many roles in neurite outgrowth and synaptic facilitation in Aplysia neurons. However, the binding property of Aplysia ARF1 by ApSec7 isoforms has not been examined. In this study, we found that the cloned Aplysia ARF1 (ApARF1) protein only localized to the Golgi complex when it was expressed alone in HEK293T cells; however, if ApARF1 was co-expressed with plasma membrane-targeted ApSec7, it localized to both the plasma membrane and the Golgi complex via association with the Sec7 domain of ApSec7. Moreover, in HEK293T cells expressing both ApARF1 and another Sec7 isoform, ApSec7(VPKIS), the pleckstrin homology domain of ApSec7(VPKIS) associated with ApARF1, resulting in its localization to the Golgi complex. Overall, we propose a model in which ApSec7(VPKIS) activates ApARF1 in the Golgi complex, while ApSec7 recruits ApARF1 to the plasma membrane where it activates ApARF1/6 downstream signaling.
Keywords: ARF1; ARF6; ApSec7; ApSec7(VPKIS); Aplysia.
Figures
Similar articles
-
Activation of Aplysia ARF6 induces neurite outgrowth and is sequestered by the overexpression of the PH domain of Aplysia Sec7 proteins.Neurobiol Learn Mem. 2017 Feb;138:31-38. doi: 10.1016/j.nlm.2016.06.017. Epub 2016 Jun 21. Neurobiol Learn Mem. 2017. PMID: 27344941
-
Analysis of phosphoinositide-binding properties and subcellular localization of GFP-fusion proteins.Lipids. 2015 Apr;50(4):427-36. doi: 10.1007/s11745-015-3994-z. Epub 2015 Feb 17. Lipids. 2015. PMID: 25688026
-
Dual roles of the N-terminal coiled-coil domain of an Aplysia sec7 protein: homodimer formation and nuclear export.J Neurochem. 2016 Dec;139(6):1102-1112. doi: 10.1111/jnc.13875. Epub 2016 Dec 6. J Neurochem. 2016. PMID: 27787889
-
Localization and function of Arf family GTPases.Biochem Soc Trans. 2005 Aug;33(Pt 4):639-42. doi: 10.1042/BST0330639. Biochem Soc Trans. 2005. PMID: 16042562 Review.
-
Activation of toxin ADP-ribosyltransferases by eukaryotic ADP-ribosylation factors.Mol Cell Biochem. 1999 Mar;193(1-2):153-7. Mol Cell Biochem. 1999. PMID: 10331652 Review.
References
LinkOut - more resources
Full Text Sources
