H5N1 Influenza a Virus Replicates Productively in Pancreatic Cells and Induces Apoptosis and Pro-Inflammatory Cytokine Response
- PMID: 30460207
- PMCID: PMC6232254
- DOI: 10.3389/fcimb.2018.00386
H5N1 Influenza a Virus Replicates Productively in Pancreatic Cells and Induces Apoptosis and Pro-Inflammatory Cytokine Response
Abstract
The inflammatory response and apoptosis have been proved to have a crucial role in the pathogenesis of the influenza A virus (IAV). Previous studies indicated that while IAV commonly causes pancreatitis and pancreatic damage in naturally and experimentally infected animals, the molecular mechanisms of the pathogenesis of IAV infection are less reported. In the present study, we showed for the first time that both avian-like (α-2,3-linked) and human-like (α-2,6-linked) sialic acid (SA) receptors were expressed by the mouse pancreatic cancer cell line PAN02 and the human pancreatic cancer cell line PANC-1. Using growth kinetics experiments, we also showed that PAN02 and PANC-1 cells supported the productive replication of the H5N1 highly pathogenic avian influenza while exhibited the limited replication of IAV subtypes H1N1 and H7N2 in vitro. The in vivo infection of H5N1 in pancreatic cells was confirmed by the histopathological and immunohistochemical staining of pancreas tissue from mice. Other than H1N1 and H7N2, severe damage and extensive positive signals were observed in pancreas of H5N1 infected mice. All three virus subtypes induced apoptosis but also triggered the infected PAN02 and PANC-1 cells to release pro-inflammatory cytokines and chemokines including interferon (IFN)-α, IFN-β, IFN-γ, chemokine (C-C motif) ligand 2 (CCL2), tumor necrosis factor (TNF)-α, and interleukin (IL)-6. Notably, the subtypes of H5N1 could significantly upregulate these cytokines and chemokines in both two cells when compared with H1N1 and H7N2. The present data provide further understanding of the pathogenesis of H5N1 IAV in pancreatic cells derived from humans and mammals and may also benefit the development of new treatment against H5N1 influenza virus infection.
Keywords: H5N1 influenza A virus; apoptosis; inflammatory response; pancreatic cells; pathogenesis.
Figures






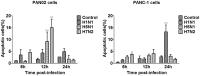


References
-
- Balachandran S., Roberts P. C., Kipperman T., Bhalla K. N., Compans R. W., Archer D. R., et al. . (2000). Alpha/beta interferons potentiate virus-induced apoptosis through activation of the FADD/Caspase-8 death signaling pathway. J. Virol. 74, 1513–1523. 10.1128/JVI.74.3.1513-1523.2000 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

