Nano-Dispersed Ziegler-Natta Catalysts for 1 μm-Sized Ultra-High Molecular Weight Polyethylene Particles
- PMID: 30460228
- PMCID: PMC6232879
- DOI: 10.3389/fchem.2018.00524
Nano-Dispersed Ziegler-Natta Catalysts for 1 μm-Sized Ultra-High Molecular Weight Polyethylene Particles
Abstract
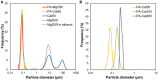
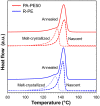
A catalytic approach to synthesize microfine ultra-high molecular weight polyethylene (UHMWPE) particles was proposed based on the exploitation of nano-sized catalysts. By utilizing MgO nanoparticles as a core material, a Ziegler-Natta-type MgO/MgCl2/TiCl4 core-shell catalyst with the particle size in a nano-range scale was prepared in a simple preparation step. The organic modification of MgO surfaces prior to catalyzation prevented agglomeration and facilitated the full dispersion of catalyst particles at a primary particle level for the first time. The nano-dispersed catalysts successfully afforded a direct access to UHMWPE having the particle size in the range of 1-2 μm at a reasonable activity. Extremely fine polymer particles yielded several advantages, especially at a significantly lower fusion temperature in compression molding.
Keywords: magnesium oxide; microfine; nano-dispersed; nano-sized catalyst; polyethylene; ultra-high molecular weight; ziegler-natta.
Figures






References
-
- Bando Y., Chammingkwan P., Terano M., Taniike T. (2018). Synthesis of ultra-high molecular weight polyethylene using MgO/MgCl2/TiCl4 core-shell catalysts. Macromol. Chem. Phys. 219:1800011 10.1002/macp.201800011 - DOI
-
- Barnetson A., Hornsby P. R. (1995). Observations on the sintering of ultra-high molecular weight polyethylene (UHMWPE) powders. J. Mater. Sci. Lett. 14, 80–84. 10.1007/BF00456553 - DOI
-
- Baumgaertner E. (1974). Ultra-High Molecular Weight Polyethylene Molding Powder and Molding Process. U.S. Patent No 3,847,888. Washington, DC; U.S. Patent and Trademark Office.
-
- Bilda D., Boehm L. (2000). Process for the Preparation of a Polymerization and Copolymerization of Ethylene to Give Ultrahigh Molecular-Weight Ethylene Polymers. U.S. Patent No 6,114,271. Washington, DC; U.S. Patent and Trademark Office.
LinkOut - more resources
Full Text Sources

