Cell Sources for Tissue Engineering Strategies to Treat Calcific Valve Disease
- PMID: 30460245
- PMCID: PMC6232262
- DOI: 10.3389/fcvm.2018.00155
Cell Sources for Tissue Engineering Strategies to Treat Calcific Valve Disease
Abstract
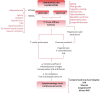
Cardiovascular calcification is an independent risk factor and an established predictor of adverse cardiovascular events. Despite concomitant factors leading to atherosclerosis and heart valve disease (VHD), the latter has been identified as an independent pathological entity. Calcific aortic valve stenosis is the most common form of VDH resulting of either congenital malformations or senile "degeneration." About 2% of the population over 65 years is affected by aortic valve stenosis which represents a major cause of morbidity and mortality in the elderly. A multifactorial, complex and active heterotopic bone-like formation process, including extracellular matrix remodeling, osteogenesis and angiogenesis, drives heart valve "degeneration" and calcification, finally causing left ventricle outflow obstruction. Surgical heart valve replacement is the current therapeutic option for those patients diagnosed with severe VHD representing more than 20% of all cardiac surgeries nowadays. Tissue Engineering of Heart Valves (TEHV) is emerging as a valuable alternative for definitive treatment of VHD and promises to overcome either the chronic oral anticoagulation or the time-dependent deterioration and reintervention of current mechanical or biological prosthesis, respectively. Among the plethora of approaches and stablished techniques for TEHV, utilization of different cell sources may confer of additional properties, desirable and not, which need to be considered before moving from the bench to the bedside. This review aims to provide a critical appraisal of current knowledge about calcific VHD and to discuss the pros and cons of the main cell sources tested in studies addressing in vitro TEHV.
Keywords: calcification; heterotopic bone formation; in vitro; tissue engineering heart valves; valve heart disease.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

