Resveratrol mitigates the oxidative stress mediated by hypoxic-ischemic brain injury in neonatal rats via Nrf2/HO-1 pathway
- PMID: 30460866
- PMCID: PMC6249550
- DOI: 10.1080/13880209.2018.1502326
Resveratrol mitigates the oxidative stress mediated by hypoxic-ischemic brain injury in neonatal rats via Nrf2/HO-1 pathway
Abstract
Context: Hypoxic-ischemic encephalopathy (HIE) has a high morbidity and mortality rate. Resveratrol possesses numerous biological properties including antioxidant, anti-inflammatory and neuroprotective activities.
Objective: The current experiment investigates the neuroprotective efficacy of resveratrol (RESV) against HIE by modulating Nrf2/HO-1 pathway in neonatal rats.
Materials and methods: Seven-day-old pups (n = 48) were divided into four groups. Group-I rats receiving 2% DMSO saline (sham), group-II rats underwent unilateral carotid artery ligation and hypoxia (92% N2 and 8% O2) for 2.5 h (hypoxia-ischemia; HI), group-III and IV rats received 20 (RESV 20 + HI) or 40 mg/kg (RESV 40 + HI; group-IV) of RESV via intraperitoneal injection (ip), respectively, for 7 days prior to HI induction.
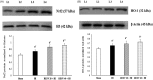
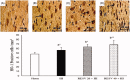
Results: Pre-treatment with RESV (20 or 40) markedly reduced (p < 0.01) the cerebral oedema (86.23-71.26 or 65.24%), infarct area (33.85-19.81 or 14.30%), lipid peroxidation products, inflammatory markers [IL-1β 186-110 or 82; IL-6 255-146 or 103; TNF-α 310-204 or 137; NF-κB 205-115 or 91) p65 subunit] and significantly restored (p < 0.01) the antioxidative status by enhancing the activities of glutathione peroxidase (GPx) 5.22-6.49 or 7.78; catalase (CAT) 51-55 or 59, superoxide dismutase (SOD) 2.5-3.05 or 3.25; through marked upregulation (p < 0.01) of heme oxygenase 1 (HO-1) 0.65-0.69 or 0.73; and nuclear factor erythroid 2 related factor 2 (Nrf2) 0.73-0.86 or 0.91.
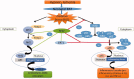
Discussion and conclusions: RESV displays its neurotherapeutic potential via upregulating the protein expression of Nrf2 and HO-1 signalling pathway and thereby attenuates oxidative stress and inflammatory response in HI-induced neonatal rats.
Keywords: Lipid peroxidation; infarct area; inflammatory markers; neurotherapeutic; oedema.
Figures




References
-
- Arteaga O, Revuelta M, Montalvo H, Cañavate ML, Alonso-Alconada D, Martinez-Ibargüen A, Hilario E, Alvarez A. 2014. Neuroprotective effect of antioxidants in neonatal rat brain after hypoxia-ischemia: microscopy: advances in scientific research and education. Badajoz, Spain: Formatex Research Center; p. 335–343.
-
- Bastianetto S, Ménard C, Quirion R. 2015. Neuroprotective action of resveratrol. Biochim Biophys Acta. 1852:1195–1201. - PubMed
-
- Block ML, Zecca L, Hong JS. 2007. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 8:57–69. - PubMed
-
- Buonocore G, Groenendaal F. 2007. Anti-oxidant strategies. Semin Fetal Neonat Med. 12:287–295. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
