Cadherin-7 mediates proper neural crest cell-placodal neuron interactions during trigeminal ganglion assembly
- PMID: 30461190
- PMCID: PMC6932833
- DOI: 10.1002/dvg.23264
Cadherin-7 mediates proper neural crest cell-placodal neuron interactions during trigeminal ganglion assembly
Abstract
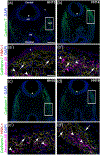
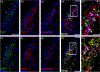
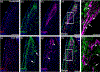
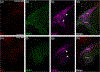
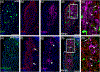
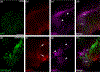
The cranial trigeminal ganglia play a vital role in the peripheral nervous system through their relay of sensory information from the vertebrate head to the brain. These ganglia are generated from the intermixing and coalescence of two distinct cell populations: cranial neural crest cells and placodal neurons. Trigeminal ganglion assembly requires the formation of cadherin-based adherens junctions within the neural crest cell and placodal neuron populations; however, the molecular composition of these adherens junctions is still unknown. Herein, we aimed to define the spatio-temporal expression pattern and function of Cadherin-7 during early chick trigeminal ganglion formation. Our data reveal that Cadherin-7 is expressed exclusively in migratory cranial neural crest cells and is absent from trigeminal neurons. Using molecular perturbation experiments, we demonstrate that modulation of Cadherin-7 in neural crest cells influences trigeminal ganglion assembly, including the organization of neural crest cells and placodal neurons within the ganglionic anlage. Moreover, alterations in Cadherin-7 levels lead to changes in the morphology of trigeminal neurons. Taken together, these findings provide additional insight into the role of cadherin-based adhesion in trigeminal ganglion formation, and, more broadly, the molecular mechanisms that orchestrate the cellular interactions essential for cranial gangliogenesis.
Keywords: Cadherin-7; neural crest; placodal neurons; trigeminal ganglion.
© 2018 Wiley Periodicals, Inc.
Figures
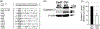

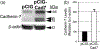

References
-
- Baker CV, & Bronner-Fraser M (2001). Vertebrate cranial placodes I. Embryonic induction. Developmental Biology, 232, 1–61. - PubMed
-
- Breau MA, & Schneider-Maunoury S (2015). Cranial placodes: Models for exploring the multi-facets of cell adhesion in epithelial rearrangement, collective migration and neuronal movements. Developmental Biology, 401, 25–36. - PubMed
-
- Bronner-Fraser M (1986). Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Developmental Biology, 115, 44–55. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

