Targeting PRMT5/Akt signalling axis prevents human lung cancer cell growth
- PMID: 30461193
- PMCID: PMC6349228
- DOI: 10.1111/jcmm.14036
Targeting PRMT5/Akt signalling axis prevents human lung cancer cell growth
Abstract
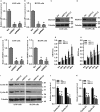
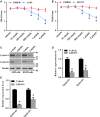
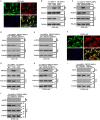
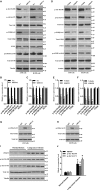
The emerging evidence reveals that protein arginine methyltransferase 5 (PRMT5) is involved in regulation of tumour cell proliferation and cancer development. Nevertheless, the exact role of PRMT5 in human lung cancer cell proliferation and the underlying molecular mechanism remains largely obscure. Here, we showed that PRMT5 was highly expressed in human lung cancer cells and lung cancer tissues. Furthermore, we generated PRMT5 stable knockdown cell lines (A549 and H1299 cells) and explored the functions of PRMT5 in lung cancer cell proliferation. We found that the down-regulation of PRMT5 by shRNA or the inhibition of PRMT5 by specific inhibitor GSK591 dramatically suppressed cyclin E1 and cyclin D1 expression and cell proliferation. Moreover, we uncovered that PRMT5 promoted lung cancer cell proliferation via regulation of Akt activation. PRMT5 was directly co-localized and interacted with Akt, but not PTEN and mTOR. Down-regulation or inhibition of PRMT5 markedly reduced Akt phosphorylation at Thr308 and Ser473, whereas the expression of PTEN and mTOR phosphorylation was unchanged, indicating that PRMT5 was an important upstream regulator of Akt and induced lung cancer cell proliferation. Altogether, our results indicate that PRMT5 promotes human lung cancer cell proliferation through direct interaction with Akt and regulation of Akt activity. Our findings also suggest that targeting PRMT5 may have therapeutic potential for treatment of human lung cancer.
Keywords: Akt; PRMT5; PTEN; lung cancer; mTOR; proliferation.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69‐90. - PubMed
-
- Kanda M, Shimizu D, Fujii T, et al. Protein arginine methyltransferase 5 is associated with malignant phenotype and peritoneal metastasis in gastric cancer. Int J Oncol. 2016;49:1195‐1202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

