Pancreatic fibroblast growth factor 21 protects against type 2 diabetes in mice by promoting insulin expression and secretion in a PI3K/Akt signaling-dependent manner
- PMID: 30461198
- PMCID: PMC6349243
- DOI: 10.1111/jcmm.14007
Pancreatic fibroblast growth factor 21 protects against type 2 diabetes in mice by promoting insulin expression and secretion in a PI3K/Akt signaling-dependent manner
Abstract
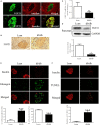
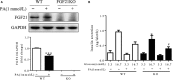
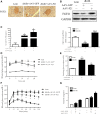
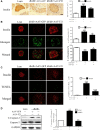
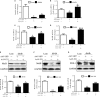
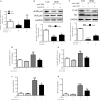
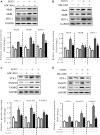
Fibroblast growth factor 21 (FGF21) is important in glucose, lipid homeostasis and insulin sensitivity. However, it remains unknown whether FGF21 is involved in insulin expression and secretion that are dysregulated in type 2 diabetes mellitus (T2DM). In this study, we found that FGF21 was down-regulated in pancreatic islets of db/db mice, a mouse model of T2DM, along with decreased insulin expression, suggesting the possible involvement of FGF21 in maintaining insulin homeostasis and islet β-cell function. Importantly, FGF21 knockout exacerbated palmitate-induced islet β-cell failure and suppression of glucose-stimulated insulin secretion (GSIS). Pancreatic FGF21 overexpression significantly increased insulin expression, enhanced GSIS, improved islet morphology and reduced β-cell apoptosis in db/db mice. Mechanistically, FGF21 promoted expression of insulin gene transcription factors and soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) proteins, the major regulators of insulin secretion, as well as activating phosphatidylinositol 3-kinase (PI3K)/Akt signaling in islets of db/db mice. In addition, pharmaceutical inhibition of PI3K/Akt signaling effectively suppressed FGF21-induced expression of insulin gene transcription factors and SNARE proteins, suggesting an essential role of PI3K/Akt signaling in FGF21-induced insulin expression and secretion. Taken together, our results demonstrate a protective role of pancreatic FGF21 in T2DM mice through inducing PI3K/Akt signaling-dependent insulin expression and secretion.
Keywords: FGF21; diabetes; insulin; pancreatic β-cell.
© 2018 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures

Similar articles
-
Loss of fibroblast growth factor 21 action induces insulin resistance, pancreatic islet hyperplasia and dysfunction in mice.Cell Death Dis. 2015 Mar 26;6(3):e1707. doi: 10.1038/cddis.2015.80. Cell Death Dis. 2015. PMID: 25811804 Free PMC article.
-
Fibroblast growth factor 21 protects against lipotoxicity-induced pancreatic β-cell dysfunction via regulation of AMPK signaling and lipid metabolism.Clin Sci (Lond). 2019 Oct 15;133(19):2029-2044. doi: 10.1042/CS20190093. Clin Sci (Lond). 2019. PMID: 31654570
-
Circulating LncRNAs Analysis in Patients with Type 2 Diabetes Reveals Novel Genes Influencing Glucose Metabolism and Islet β-Cell Function.Cell Physiol Biochem. 2018;46(1):335-350. doi: 10.1159/000488434. Epub 2018 Mar 22. Cell Physiol Biochem. 2018. PMID: 29590649
-
The potential effect of metformin on fibroblast growth factor 21 in type 2 diabetes mellitus (T2DM).Inflammopharmacology. 2023 Aug;31(4):1751-1760. doi: 10.1007/s10787-023-01255-4. Epub 2023 Jun 19. Inflammopharmacology. 2023. PMID: 37337094 Review.
-
The PI3K/Akt signaling axis and type 2 diabetes mellitus (T2DM): From mechanistic insights into possible therapeutic targets.Cell Biol Int. 2024 Aug;48(8):1049-1068. doi: 10.1002/cbin.12189. Epub 2024 May 29. Cell Biol Int. 2024. PMID: 38812089 Review.
Cited by
-
Carnosic Acid Protects INS-1 β-Cells against Streptozotocin-Induced Damage by Inhibiting Apoptosis and Improving Insulin Secretion and Glucose Uptake.Molecules. 2022 Mar 24;27(7):2102. doi: 10.3390/molecules27072102. Molecules. 2022. PMID: 35408495 Free PMC article.
-
Differential disruption on glucose and insulin metabolism in two rat models of diet-induced obesity, based on carbohydrates or lipids.Mol Cell Biochem. 2023 Nov;478(11):2481-2488. doi: 10.1007/s11010-023-04677-4. Epub 2023 Mar 3. Mol Cell Biochem. 2023. PMID: 36867342
-
FGF21: An Emerging Therapeutic Target for Non-Alcoholic Steatohepatitis and Related Metabolic Diseases.Front Endocrinol (Lausanne). 2020 Dec 14;11:601290. doi: 10.3389/fendo.2020.601290. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33381084 Free PMC article. Review.
-
Sex-dependent effects of FGF21 on HPA axis regulation and adrenal regeneration after Cushing syndrome in mice.Mol Metab. 2025 Jun;96:102122. doi: 10.1016/j.molmet.2025.102122. Epub 2025 Mar 26. Mol Metab. 2025. PMID: 40154841 Free PMC article.
-
The Association Between FGF21 and Diabetic Erectile Dysfunction: Evidence from Clinical and Animal Studies.Front Endocrinol (Lausanne). 2022 Jun 15;13:874796. doi: 10.3389/fendo.2022.874796. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36213282 Free PMC article.
References
-
- Ors D, Eroglu Altinova A, Yalcin MM, et al. Fibroblast growth factor 21 and its relationship with insulin sensitivity in first‐degree relatives of patients with type 2 diabetes mellitus. Endokrynol Pol. 2016;67:260‐264. - PubMed
-
- Leahy JL, Hirsch IB, Peterson KA, Schneider D. Targeting β‐cell function early in the course of therapy for type 2 diabetes mellitus. J Clin Endocrinol Metab. 2010;95:4206‐4216. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

