The PPARα agonist fenofibrate attenuates disruption of dopamine function in a maternal immune activation rat model of schizophrenia
- PMID: 30461214
- PMCID: PMC6488881
- DOI: 10.1111/cns.13087
The PPARα agonist fenofibrate attenuates disruption of dopamine function in a maternal immune activation rat model of schizophrenia
Abstract
Aims: Prenatal maternal immune activation (MIA) is associated with a risk to develop schizophrenia and affects dopamine systems in the ventral tegmental area (VTA), key region in the neurobiology of psychoses. Considering the well-described sex differences in schizophrenia, we investigated whether sex affects MIA impact on dopamine system and on schizophrenia-related behavioral phenotype. Furthermore, considering peroxisome proliferator-activated receptor-α (PPARα) expression in the CNS as well as its anti-inflammatory and neuroprotective properties, we tested if PPARα activation by prenatal treatment with a clinically available fibrate (fenofibrate) may mitigate MIA-related effects.
Methods: We induced MIA in rat dams with polyriboinosinic-polyribocytidylic acid (Poly I:C) and assessed prepulse inhibition and dopamine neuron activity in the VTA by means of electrophysiological recordings in male and female preweaned and adult offspring.
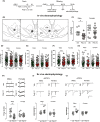
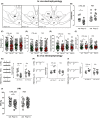
Results: Poly I:C-treated males displayed prepulse inhibition deficits, reduced number and firing rate of VTA dopamine neurons, and paired-pulse facilitation of inhibitory and excitatory synapses. Prenatal fenofibrate administration attenuated detrimental effects induced by MIA on both the schizophrenia-like behavioral phenotype and dopamine transmission in male offspring.
Conclusion: Our study confirms previous evidence that females are less susceptible to MIA and highlights PPARα as a potential target for treatments in schizophrenia.
Keywords: dopamine neurons; electrophysiology; maternal immune activation; peroxisome proliferator-activated receptor-alpha; schizophrenia; sex differences.
© 2018 The Authors. CNS Neuroscience & Therapeutics Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
PPARα Signaling: A Candidate Target in Psychiatric Disorder Management.Biomolecules. 2022 May 20;12(5):723. doi: 10.3390/biom12050723. Biomolecules. 2022. PMID: 35625650 Free PMC article. Review.
-
PPARα modulation of mesolimbic dopamine transmission rescues depression-related behaviors.Neuropharmacology. 2016 Nov;110(Pt A):251-259. doi: 10.1016/j.neuropharm.2016.07.024. Epub 2016 Jul 22. Neuropharmacology. 2016. PMID: 27457507
-
The PPARα agonist fenofibrate reduces the cytokine imbalance in a maternal immune activation model of schizophrenia.Eur J Pharmacol. 2023 Dec 15;961:176172. doi: 10.1016/j.ejphar.2023.176172. Epub 2023 Nov 6. Eur J Pharmacol. 2023. PMID: 37939988
-
Δ9-Tetrahydrocannabinol During Adolescence Attenuates Disruption of Dopamine Function Induced in Rats by Maternal Immune Activation.Front Behav Neurosci. 2019 Sep 6;13:202. doi: 10.3389/fnbeh.2019.00202. eCollection 2019. Front Behav Neurosci. 2019. PMID: 31551729 Free PMC article.
-
Maternal Immune Activation by Poly I:C as a preclinical Model for Neurodevelopmental Disorders: A focus on Autism and Schizophrenia.Neurosci Biobehav Rev. 2020 Jun;113:546-567. doi: 10.1016/j.neubiorev.2020.04.012. Epub 2020 Apr 19. Neurosci Biobehav Rev. 2020. PMID: 32320814 Review.
Cited by
-
Repurposing Peroxisome Proliferator-Activated Receptor Agonists in Neurological and Psychiatric Disorders.Pharmaceuticals (Basel). 2021 Oct 8;14(10):1025. doi: 10.3390/ph14101025. Pharmaceuticals (Basel). 2021. PMID: 34681249 Free PMC article. Review.
-
Reversing the Psychiatric Effects of Neurodevelopmental Cannabinoid Exposure: Exploring Pharmacotherapeutic Interventions for Symptom Improvement.Int J Mol Sci. 2021 Jul 23;22(15):7861. doi: 10.3390/ijms22157861. Int J Mol Sci. 2021. PMID: 34360626 Free PMC article. Review.
-
NEP1-40 Regulates the Development of Hippocampal Neural Stem Cells in Schizophrenic Mice.Curr Med Sci. 2025 Aug;45(4):930-943. doi: 10.1007/s11596-025-00078-4. Epub 2025 Jul 11. Curr Med Sci. 2025. PMID: 40643877
-
Is There a Future for PPARs in the Treatment of Neuropsychiatric Disorders?Molecules. 2020 Feb 27;25(5):1062. doi: 10.3390/molecules25051062. Molecules. 2020. PMID: 32120979 Free PMC article. Review.
-
PPARα Signaling: A Candidate Target in Psychiatric Disorder Management.Biomolecules. 2022 May 20;12(5):723. doi: 10.3390/biom12050723. Biomolecules. 2022. PMID: 35625650 Free PMC article. Review.
References
-
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003;28(10):1778‐1789. - PubMed
-
- Abi‐Dargham A, Gil R, Krystal J, et al. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. AJ Psychiatry. 1998;155(6):761‐767. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

