Reversible Control of Spacing in Charged Lamellar Membrane Hydrogels by Hydrophobically Mediated Tethering with Symmetric and Asymmetric Double-End-Anchored Poly(ethylene glycol)s
- PMID: 30461259
- PMCID: PMC6485416
- DOI: 10.1021/acsami.8b16456
Reversible Control of Spacing in Charged Lamellar Membrane Hydrogels by Hydrophobically Mediated Tethering with Symmetric and Asymmetric Double-End-Anchored Poly(ethylene glycol)s
Abstract
Complex materials often achieve their remarkable functional properties by hierarchical assembly of building blocks via competing and/or synergistic interactions. Here, we describe the properties of new double-end-anchored poly(ethylene glycol)s (DEA-PEGs)-macromolecules designed to impart hydrophobically mediated tethering attractions between charged lipid membranes. We synthesized DEA-PEGs (MW 2000 (2K) and 4.6K) with two double-tail (symmetric) or a double-tail and a single-tail (asymmetric) hydrophobic end anchors and characterized their equilibrium and kinetic properties using small-angle X-ray scattering. Control multilayer membranes without and with PEG lipid (i.e., single-end-anchored PEG) swelled continuously, with the interlayer spacing increasing between 30 and 90 wt % water content due to electrostatic as well as, in the case of PEG lipid, steric repulsion. In contrast, interlayer spacings in lamellar membrane hydrogels containing DEA-PEGs expanded over a limited water dilution range and reached a "locked" state, which displayed a near constant membrane wall-to-wall spacing (δw) with further increases in water content. Remarkably, the locked state displays a simple relation to the PEG radius of gyration δw ≈ 1.6 RG for both 2K and 4.6K PEG. Nevertheless, δw being considerably less than the physical size of PEG (2(5/3)1/2 RG) is highly unexpected and implies that, compared to free PEG, anchoring of the PEG tether at both ends leads to a considerable distortion of the PEG conformation confined between layers. Significantly, the lamellar hydrogel may be designed to reversibly transition from a locked to an unlocked (membrane unbinding) state by variations in the DEA-PEG concentration, controlling the strength of the interlayer attractions due to bridging conformations. The findings with DEA-PEGs have broad implications for hydrophobic-mediated assembly of lipid- or surfactant-coated building blocks with distinct shape and size, at predictable spacing, in aqueous environments.
Keywords: PEG lipid; hydrophobic mediated tethering; lamellar phase; lipid bilayer; lipid membrane; self-assembly.
Figures



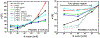
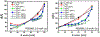

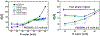

Similar articles
-
Assembly of Building Blocks by Double-End-Anchored Polymers in the Dilute Regime Mediated by Hydrophobic Interactions at Controlled Distances.ACS Appl Mater Interfaces. 2020 Oct 14;12(41):45728-45743. doi: 10.1021/acsami.0c10972. Epub 2020 Oct 5. ACS Appl Mater Interfaces. 2020. PMID: 32960036 Free PMC article.
-
Size-selective protein adsorption to polystyrene surfaces by self-assembled grafted poly(ethylene glycols) with varied chain lengths.Langmuir. 2005 Sep 13;21(19):8774-84. doi: 10.1021/la051049r. Langmuir. 2005. PMID: 16142960
-
Insertion stability of poly(ethylene glycol)-cholesteryl-based lipid anchors in liposome membranes.Eur J Pharm Biopharm. 2016 Jun;103:51-61. doi: 10.1016/j.ejpb.2016.03.023. Epub 2016 Mar 22. Eur J Pharm Biopharm. 2016. PMID: 27016212
-
Thermoresponsive physical hydrogels of poly(lactic acid)/poly(ethylene glycol) stereoblock copolymers tuned by stereostructure and hydrophobic block sequence.Soft Matter. 2016 May 18;12(20):4628-37. doi: 10.1039/c6sm00517a. Soft Matter. 2016. PMID: 27121732
-
Morphology of Photopolymerized End-linked Poly(ethylene glycol) Hydrogels by Small Angle X-ray Scattering.Macromolecules. 2010 Aug 24;43(16):6861-6870. doi: 10.1021/ma101070s. Macromolecules. 2010. PMID: 21403767 Free PMC article.
Cited by
-
Nucleated synthetic cells with genetically driven intercompartment communication.Proc Natl Acad Sci U S A. 2024 Sep 3;121(36):e2404790121. doi: 10.1073/pnas.2404790121. Epub 2024 Aug 26. Proc Natl Acad Sci U S A. 2024. PMID: 39186653 Free PMC article.
-
Engineering a nanoscale liposome-in-liposome for in situ biochemical synthesis and multi-stage release.Nat Chem. 2024 Oct;16(10):1612-1620. doi: 10.1038/s41557-024-01584-z. Epub 2024 Jul 15. Nat Chem. 2024. PMID: 39009794 Free PMC article.
-
Assembly of Building Blocks by Double-End-Anchored Polymers in the Dilute Regime Mediated by Hydrophobic Interactions at Controlled Distances.ACS Appl Mater Interfaces. 2020 Oct 14;12(41):45728-45743. doi: 10.1021/acsami.0c10972. Epub 2020 Oct 5. ACS Appl Mater Interfaces. 2020. PMID: 32960036 Free PMC article.
References
-
- Fratzl P; Speck T; Gorb S: Function by Internal Structure. Bioinspiration Biomimetics 2016, 11, 060301. - PubMed
-
- Mishnaevsky L; Tsapatsis M: Hierarchical Materials: Background and Perspectives. MRS Bull. 2016, 41, 661–664. DOI: 10.1557/mrs.2016.189. - DOI
-
- Lakes R: Materials with Structural Hierarchy. Nature 1993, 361, 511 DOI: 10.1038/361511a0. - DOI
-
- Fratzl P; Weinkamer R: Nature’s Hierarchical Materials. Prog. Mater. Sci. 2007, 52, 1263–1334. DOI: 10.1016/j.pmatsci.2007.06.001. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

