Na+/Ca2+ exchanger overexpression in smooth muscle augments cytosolic Ca2+ in femoral arteries of living mice
- PMID: 30461304
- PMCID: PMC6397384
- DOI: 10.1152/ajpheart.00185.2018
Na+/Ca2+ exchanger overexpression in smooth muscle augments cytosolic Ca2+ in femoral arteries of living mice
Erratum in
-
Corrigendum.Am J Physiol Heart Circ Physiol. 2019 Mar 1;316(3):H762-H763. doi: 10.1152/ajpheart.zh4-2706-corr-2019. Am J Physiol Heart Circ Physiol. 2019. PMID: 30839230 Free PMC article. No abstract available.
Abstract
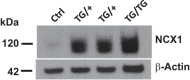
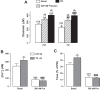
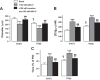
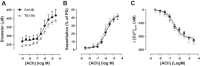
Plasma membrane Na+/Ca2+ exchanger-1 (NCX1) helps regulate the cytosolic Ca2+ concentration ([Ca2+]CYT) in arterial myocytes. NCX1 mediates both Ca2+ entry and exit and tends to promote net Ca2+ entry in partially constricted arteries. Mean blood pressure (telemetry) is elevated by ≈10 mmHg in transgenic (TG) mice that overexpress NCX1 specifically in smooth muscle. We tested the hypothesis that NCX1 overexpression mediates Ca2+ gain and elevated [Ca2+]CYT in exposed femoral arteries that also express the Ca2+ biosensor exogenous myosin light chain kinase. [Ca2+]CYT and the NCX1-dependent (SEA0400-sensitive) component, ≈15% of total basal constriction in controls, were increased in TG arteries, but constrictions to phenylephrine and ANG II were comparable in TG and control arteries. Normalized phenylephrine dose-response curves and constriction to 30 and 300 ng/kg iv ANG II were virtually identical in control and TG arteries. ANG II-evoked constrictions, superimposed on elevated basal tone, accounted for the larger blood pressure responses to ANG II in TG arteries. TG and control mouse arteries fit the same pCa-constriction relationship over a wide range of pCa (≈125-500 nM). Vasodilation to acetylcholine, normalized to passive diameter, was also comparable in TG and control arteries, implying normal endothelial function. TG artery Na+ nitroprusside (nitric oxide donor)-induced dilations were, however, shifted to lower Na+ nitroprusside concentrations, indicating that TG myocyte vasodilator mechanisms were augmented. Maximum arterial dilation was comparable in TG and control mice, although passive diameter was ≈6-7% smaller in TG mice. The changes in TG arteries were apparently largely functional rather than structural, despite the congenital hypertension. NEW & NOTEWORTHY Smooth muscle Na+/Ca2+ exchanger-1 transgene overexpression (TG mice) increases femoral artery basal cytosolic Ca2+ concentration ([Ca2+]CYT) and tone in vivo and raises blood pressure. Arterial constriction to phenylephrine and angiotensin II are normal but superimposed on the augmented basal [Ca2+]CYT and tone (constriction) in TG mouse arteries. Similar effects in resistance arteries would explain the elevated blood pressure. Acetylcholine-induced vasodilation is unimpaired, implying a normal endothelium, but TG arteries are hypersensitive to sodium nitroprusside.
Keywords: SEA0400, sodium nitroprusside; arterial tone; vasodilation.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures






Similar articles
-
NO-induced vasodilation correlates directly with BP in smooth muscle-Na/Ca exchanger-1-engineered mice: elevated BP does not attenuate endothelial function.Am J Physiol Heart Circ Physiol. 2021 Jan 1;320(1):H221-H237. doi: 10.1152/ajpheart.00487.2020. Epub 2020 Oct 30. Am J Physiol Heart Circ Physiol. 2021. PMID: 33124883 Free PMC article.
-
Knockout of Na+/Ca2+ exchanger in smooth muscle attenuates vasoconstriction and L-type Ca2+ channel current and lowers blood pressure.Am J Physiol Heart Circ Physiol. 2010 May;298(5):H1472-83. doi: 10.1152/ajpheart.00964.2009. Epub 2010 Feb 19. Am J Physiol Heart Circ Physiol. 2010. PMID: 20173044 Free PMC article.
-
Arterial α2-Na+ pump expression influences blood pressure: lessons from novel, genetically engineered smooth muscle-specific α2 mice.Am J Physiol Heart Circ Physiol. 2015 Sep;309(5):H958-68. doi: 10.1152/ajpheart.00430.2015. Epub 2015 Jul 24. Am J Physiol Heart Circ Physiol. 2015. PMID: 26209057 Free PMC article.
-
New insights into the contribution of arterial NCX to the regulation of myogenic tone and blood pressure.Adv Exp Med Biol. 2013;961:329-43. doi: 10.1007/978-1-4614-4756-6_28. Adv Exp Med Biol. 2013. PMID: 23224892 Review.
-
[Mechanisms for linking high salt intake to vascular tone: role of Na(+) pump and Na(+)/Ca²(+) exchanger coupling].Yakugaku Zasshi. 2010 Nov;130(11):1399-405. doi: 10.1248/yakushi.130.1399. Yakugaku Zasshi. 2010. PMID: 21048395 Review. Japanese.
Cited by
-
Pathophysiology and pathogenic mechanisms of pulmonary hypertension: role of membrane receptors, ion channels, and Ca2+ signaling.Physiol Rev. 2023 Jul 1;103(3):1827-1897. doi: 10.1152/physrev.00030.2021. Epub 2022 Nov 24. Physiol Rev. 2023. PMID: 36422993 Free PMC article. Review.
-
The Calcium Signaling Mechanisms in Arterial Smooth Muscle and Endothelial Cells.Compr Physiol. 2021 Apr 1;11(2):1831-1869. doi: 10.1002/cphy.c200030. Compr Physiol. 2021. PMID: 33792900 Free PMC article.
-
Mathematical modeling and biochemical analysis support partially ordered calmodulin-myosin light chain kinase binding.iScience. 2023 Feb 4;26(4):106146. doi: 10.1016/j.isci.2023.106146. eCollection 2023 Apr 21. iScience. 2023. PMID: 36968084 Free PMC article.
-
The Contribution of Gut Microbiota and Endothelial Dysfunction in the Development of Arterial Hypertension in Animal Models and in Humans.Int J Mol Sci. 2022 Mar 28;23(7):3698. doi: 10.3390/ijms23073698. Int J Mol Sci. 2022. PMID: 35409057 Free PMC article. Review.
-
NO-induced vasodilation correlates directly with BP in smooth muscle-Na/Ca exchanger-1-engineered mice: elevated BP does not attenuate endothelial function.Am J Physiol Heart Circ Physiol. 2021 Jan 1;320(1):H221-H237. doi: 10.1152/ajpheart.00487.2020. Epub 2020 Oct 30. Am J Physiol Heart Circ Physiol. 2021. PMID: 33124883 Free PMC article.
References
-
- Aalkjaer C, Heagerty AM, Petersen KK, Swales JD, Mulvany MJ. Evidence for increased media thickness, increased neuronal amine uptake, and depressed excitation−contraction coupling in isolated resistance vessels from essential hypertensives. Circ Res 61: 181–186, 1987. doi:10.1161/01.RES.61.2.181. - DOI - PubMed
-
- Baryshnikov SG, Pulina MV, Zulian A, Linde CI, Golovina VA. Orai1, a critical component of store-operated Ca2+ entry, is functionally associated with Na+/Ca2+ exchanger and plasma membrane Ca2+ pump in proliferating human arterial myocytes. Am J Physiol Cell Physiol 297: C1103–C1112, 2009. doi:10.1152/ajpcell.00283.2009. - DOI - PMC - PubMed
-
- Berne RM, Levy MN. Cardiovascular Physiology. St. Louis, MO: Mosby, 2001, p. 115–153.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

