Intersegmental coordination patterns are differently affected in Parkinson's disease and cerebellar ataxia
- PMID: 30461364
- PMCID: PMC6397403
- DOI: 10.1152/jn.00788.2017
Intersegmental coordination patterns are differently affected in Parkinson's disease and cerebellar ataxia
Abstract
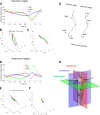
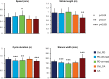
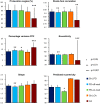
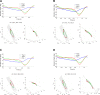
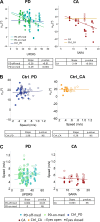
The law of intersegmental coordination (Borghese et al. 1996) may be altered in pathological conditions. Here we investigated the contribution of the basal ganglia (BG) and the cerebellum to lower limb intersegmental coordination by inspecting the plane's orientation and other parameters pertinent to this law in patients with idiopathic Parkinson's disease (PD) or cerebellar ataxia (CA). We also applied a mathematical model that successfully accounts for the intersegmental law of coordination observed in control subjects (Barliya et al. 2009). In the present study, we compared the planarity index (PI), covariation plane (CVP) orientation, and CVP orientation predicted by the model in 11 PD patients, 8 CA patients, and two groups of healthy subjects matched for age, height, weight, and gender to each patient group (Ctrl_PD and Ctrl_CA). Controls were instructed to alter their gait speed to match those of their respective patient group. PD patients were examined after overnight withdrawal of anti-parkinsonian medications (PD-off-med) and then on medication (PD-on-med). PI was above 96% in all gait conditions in all groups suggesting that the law of intersegmental coordination is preserved in both BG and cerebellar pathology. However, the measured and predicted CVP orientations rotated in PD-on-med and PD-off-med compared with Ctrl_PD and in CA vs. Ctrl_CA. These rotations caused by PD and CA were in opposite directions suggesting differences in the roles of the BG and cerebellum in intersegmental coordination during human locomotion. NEW & NOTEWORTHY Kinematic and muscular synergies may have a role in overcoming motor redundancies, which may be reflected in intersegmental covariation. Basal ganglia and cerebellar networks were suggested to be involved in crafting and modulating synergies. We thus compared intersegmental coordination in Parkinson's disease and cerebellar disease patients and found opposite effects in some aspects. Further research integrating muscle activities as well as biomechanical and neural control modeling are needed to account for these findings.
Keywords: Parkinson’s disease; cerebellar ataxia; intersegmental coordination.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



Similar articles
-
Walking in circles: navigation deficits from Parkinson's disease but not from cerebellar ataxia.Neuroscience. 2011 Sep 8;190:177-83. doi: 10.1016/j.neuroscience.2011.06.020. Epub 2011 Jun 14. Neuroscience. 2011. PMID: 21704129 Free PMC article.
-
Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia.Brain. 2003 Oct;126(Pt 10):2312-22. doi: 10.1093/brain/awg230. Epub 2003 Jun 23. Brain. 2003. PMID: 12821507
-
Locomotor patterns in cerebellar ataxia.J Neurophysiol. 2014 Dec 1;112(11):2810-21. doi: 10.1152/jn.00275.2014. Epub 2014 Sep 3. J Neurophysiol. 2014. PMID: 25185815
-
Cortico-basal ganglia and cortico-cerebellar circuits in Parkinson's disease: pathophysiology or compensation?Behav Neurosci. 2013 Apr;127(2):222-36. doi: 10.1037/a0031226. Epub 2012 Dec 17. Behav Neurosci. 2013. PMID: 23244290 Review.
-
Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease.Mov Disord. 2008;23 Suppl 3:S548-59. doi: 10.1002/mds.22062. Mov Disord. 2008. PMID: 18781672 Review.
Cited by
-
Human arm redundancy: a new approach for the inverse kinematics problem.R Soc Open Sci. 2024 Feb 28;11(2):231036. doi: 10.1098/rsos.231036. eCollection 2024 Feb. R Soc Open Sci. 2024. PMID: 38420627 Free PMC article.
-
Muscle Synergies in Parkinson's Disease.Sensors (Basel). 2020 Jun 5;20(11):3209. doi: 10.3390/s20113209. Sensors (Basel). 2020. PMID: 32517013 Free PMC article. Review.
-
Trend change analysis of postural balance in Parkinson's disease discriminates between medication state.J Neuroeng Rehabil. 2024 Jun 28;21(1):112. doi: 10.1186/s12984-024-01411-z. J Neuroeng Rehabil. 2024. PMID: 38943208 Free PMC article.
-
Lateral weight transfer deficits reveal balance vulnerability in early-stage Parkinson's Disease during trip-perturbed walking.Res Sq [Preprint]. 2025 May 8:rs.3.rs-6298735. doi: 10.21203/rs.3.rs-6298735/v1. Res Sq. 2025. PMID: 40386395 Free PMC article. Preprint.
-
Submovements in manual tracking: people with Parkinson's disease produce more submovements than age-matched controls.J Neuroeng Rehabil. 2025 Mar 6;22(1):51. doi: 10.1186/s12984-025-01592-1. J Neuroeng Rehabil. 2025. PMID: 40050876 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

