T5 exonuclease-dependent assembly offers a low-cost method for efficient cloning and site-directed mutagenesis
- PMID: 30462336
- PMCID: PMC6379645
- DOI: 10.1093/nar/gky1169
T5 exonuclease-dependent assembly offers a low-cost method for efficient cloning and site-directed mutagenesis
Abstract
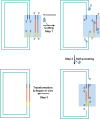
The assembly of DNA fragments with homologous arms is becoming popular in routine cloning. For an in vitro assembly reaction, a DNA polymerase is often used either alone for its 3'-5' exonuclease activity or together with a 5'-3' exonuclease for its DNA polymerase activity. Here, we present a 'T5 exonuclease DNA assembly' (TEDA) method that only uses a 5'-3' exonuclease. DNA fragments with short homologous ends were treated by T5 exonuclease and then transformed into Escherichia coli to produce clone colonies. The cloning efficiency was similar to that of the commercial In-Fusion method employing a proprietary DNA polymerase, but higher than that of the Gibson method utilizing T5 exonuclease, Phusion DNA polymerase, and DNA ligase. It also assembled multiple DNA fragments and did simultaneous site-directed mutagenesis at multiple sites. The reaction mixture was simple, and each reaction used 0.04 U of T5 exonuclease that cost 0.25 US cents. The simplicity, cost effectiveness, and cloning efficiency should promote its routine use, especially for labs with a budget constraint. TEDA may trigger further development of DNA assembly methods that employ single exonucleases.
© The Author(s) 2018. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





References
-
- Ellis T., Adie T., Baldwin G.S.. DNA assembly for synthetic biology: from parts to pathways and beyond. Integr. Biol. 2011; 3:109–118. - PubMed
-
- Casini A., Storch M., Baldwin G.S., Ellis T.. Bricks and blueprints: methods and standards for DNA assembly. Nat. Rev. Mol. Cell Biol. 2015; 16:568–576. - PubMed
-
- Fu J., Bian X., Hu S., Wang H., Huang F., Seibert P.M., Plaza A., Xia L., Müller R., Stewart A.F.. Full-length RecE enhances linear-linear homologous recombination and facilitates direct cloning for bioprospecting. Nat. Biotechnol. 2012; 30:440. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

