The bystander effect contributes to the accumulation of senescent cells in vivo
- PMID: 30462359
- PMCID: PMC6351849
- DOI: 10.1111/acel.12848
The bystander effect contributes to the accumulation of senescent cells in vivo
Abstract
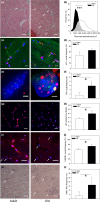
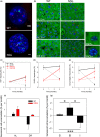
Senescent cells accumulate with age in multiple tissues and may cause age-associated disease and functional decline. In vitro, senescent cells induce senescence in bystander cells. To see how important this bystander effect may be for accumulation of senescent cells in vivo, we xenotransplanted senescent cells into skeletal muscle and skin of immunocompromised NSG mice. 3 weeks after the last transplantation, mouse dermal fibroblasts and myofibres displayed multiple senescence markers in the vicinity of transplanted senescent cells, but not where non-senescent or no cells were injected. Adjacent to injected senescent cells, the magnitude of the bystander effect was similar to the increase in senescence markers in myofibres between 8 and 32 months of age. The age-associated increase of senescence markers in muscle correlated with fibre thinning, a widely used marker of muscle aging and sarcopenia. Senescent cell transplantation resulted in borderline induction of centrally nucleated fibres and no significant thinning, suggesting that myofibre aging might be a delayed consequence of senescence-like signalling. To assess the relative importance of the bystander effect versus cell-autonomous senescence, we compared senescent hepatocyte frequencies in livers of wild-type and NSG mice under ad libitum and dietary restricted feeding. This enabled us to approximate cell-autonomous and bystander-driven senescent cell accumulation as well as the impact of immunosurveillance separately. The results suggest a significant impact of the bystander effect for accumulation of senescent hepatocytes in liver and indicate that senostatic interventions like dietary restriction may act as senolytics in immunocompetent animals.
Keywords: aging; bystander; muscle; senescence; skin.
© 2018 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- BB/K019260/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- C12161/A24009/CRUK_/Cancer Research UK/United Kingdom
- BB/C008200/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F010966/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- 24009/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources

