Dual-target MDM2/MDMX inhibitor increases the sensitization of doxorubicin and inhibits migration and invasion abilities of triple-negative breast cancer cells through activation of TAB1/TAK1/p38 MAPK pathway
- PMID: 30462562
- PMCID: PMC6605999
- DOI: 10.1080/15384047.2018.1539290
Dual-target MDM2/MDMX inhibitor increases the sensitization of doxorubicin and inhibits migration and invasion abilities of triple-negative breast cancer cells through activation of TAB1/TAK1/p38 MAPK pathway
Abstract
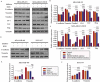
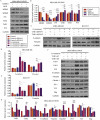
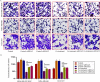
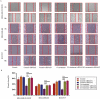
Triple-negative breast cancer (TNBC) has a poor prognosis mainly due to insensitivity or resistance to standard anthracycline- and taxane-based chemotherapy, urgently calling for new adjuvants to reverse drug resistance. Dual-target murine double minute 2 (MDM2) and murine double minute X (MDMX) inhibitor has been proved to play a critical part against cancer, particularly focusing on the tremendous potential to enhance the efficacy of doxorubicin (DOX), however little was reported in TNBC. In the present study, we investigated the synergistic antitumor effect of the MDM2/MDMX inhibitor with DOX using three TNBC cell lines, two in situ transplantation tumor models and 214 clinical samples. We observed that the MDM2/MDMX inhibitor combined with DOX could not only inhibit cell vitality and migration and invasion abilities, but also highly inhibit tumor growth in TNBC nude mice. Besides, co-treatment of MDM2/MDMX inhibitor and DOX suppressed epithelial to mesenchymal transition (EMT) through increasing the TAK1-binding protein 1 (TAB1), transforming growth factor β-activated kinase 1 (TAK1) and p38 mitogen-activated protein kinase (MAPK) expression. Small interfering RNA-mediated TAB1 knockdown induced the EMT, desensitized cells to DOX and enhanced the migration and invasion abilities. High MDM2/MDMX expression was positively associated with weak TAB1 expression in 214 TNBC tumor tissues confirmed by immumohistochemical staining and MDM2/MDMX/TAB1 expression was significantly related to TNBC patient survival. These findings indicate that dual-target MDM2/MDMX inhibitor could increase the sensitization of doxorubicin and inhibit migration and invasion abilities in TNBC cells through p38 MAPK pathway activation caused EMT suppression and hence could be useful in TNBC treatments in future.
Keywords: MDM2; MDMX; TAB1; doxorubicin resistance; invasion; migration; triple-negative breast cancer.
Figures


Similar articles
-
Recombinant Dual-target MDM2/MDMX Inhibitor Reverses Doxorubicin Resistance through Activation of the TAB1/TAK1/p38 MAPK Pathway in Wild-type p53 Multidrug-resistant Breast Cancer Cells.J Cancer. 2020 Jan 1;11(1):25-40. doi: 10.7150/jca.32765. eCollection 2020. J Cancer. 2020. PMID: 31892970 Free PMC article.
-
Context-dependent roles of MDMX (MDM4) and MDM2 in breast cancer proliferation and circulating tumor cells.Breast Cancer Res. 2019 Jan 14;21(1):5. doi: 10.1186/s13058-018-1094-8. Breast Cancer Res. 2019. PMID: 30642351 Free PMC article.
-
Cisplatin causes cell death via TAB1 regulation of p53/MDM2/MDMX circuitry.Genes Dev. 2013 Aug 15;27(16):1739-51. doi: 10.1101/gad.212258.112. Epub 2013 Aug 9. Genes Dev. 2013. PMID: 23934659 Free PMC article.
-
Targeting immune cells in tumor microenvironment in triple negative breast cancer therapy: future perspective to overcome doxorubicin resistance and toxicity.Med Oncol. 2025 Apr 4;42(5):150. doi: 10.1007/s12032-025-02712-6. Med Oncol. 2025. PMID: 40183881 Review.
-
Cellular, Structural Basis, and Recent Progress for Targeting Murine Double Minute X (MDMX) in Tumors.J Med Chem. 2024 Sep 12;67(17):14723-14741. doi: 10.1021/acs.jmedchem.4c00913. Epub 2024 Aug 26. J Med Chem. 2024. PMID: 39185935 Review.
Cited by
-
MDMX in Cancer: A Partner of p53 and a p53-Independent Effector.Biologics. 2024 Jan 31;18:61-78. doi: 10.2147/BTT.S436629. eCollection 2024. Biologics. 2024. PMID: 38318098 Free PMC article. Review.
-
LIMD1 Increases the Sensitivity of Lung Adenocarcinoma Cells to Cisplatin via the GADD45α/p38 MAPK Signaling Pathway.Front Oncol. 2020 Jul 10;10:969. doi: 10.3389/fonc.2020.00969. eCollection 2020. Front Oncol. 2020. PMID: 32754438 Free PMC article.
-
Association between TP53 mutation and high 21-gene recurrence score in estrogen receptor-positive/HER2-negative breast cancer.NPJ Breast Cancer. 2022 Feb 16;8(1):19. doi: 10.1038/s41523-022-00384-3. NPJ Breast Cancer. 2022. PMID: 35173185 Free PMC article.
-
The emerging role of microRNAs and long noncoding RNAs in drug resistance of hepatocellular carcinoma.Mol Cancer. 2019 Oct 25;18(1):147. doi: 10.1186/s12943-019-1086-z. Mol Cancer. 2019. PMID: 31651347 Free PMC article. Review.
-
Targeting MDM2-p53 interaction for breast cancer therapy.Oncol Res. 2025 Mar 19;33(4):851-861. doi: 10.32604/or.2025.058956. eCollection 2025. Oncol Res. 2025. PMID: 40191734 Free PMC article. Review.
References
-
- Giai M, Biglia N, Sismondi P. Chemoresistance in breast tumors. Eur J Gynaecol Oncol. 12;1991:359–373. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
