Macro-connectomics and microstructure predict dynamic plasticity patterns in the non-human primate brain
- PMID: 30462609
- PMCID: PMC6249000
- DOI: 10.7554/eLife.34354
Macro-connectomics and microstructure predict dynamic plasticity patterns in the non-human primate brain
Abstract
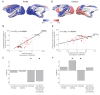
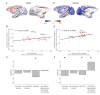
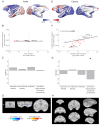
The brain displays a remarkable ability to adapt following injury by altering its connections through neural plasticity. Many of the biological mechanisms that underlie plasticity are known, but there is little knowledge as to when, or where in the brain plasticity will occur following injury. This knowledge could guide plasticity-promoting interventions and create a more accurate roadmap of the recovery process following injury. We causally investigated the time-course of plasticity after hippocampal lesions using multi-modal MRI in monkeys. We show that post-injury plasticity is highly dynamic, but also largely predictable on the basis of the functional connectivity of the lesioned region, gradients of cell densities across the cortex and the pre-lesion network structure of the brain. The ability to predict which brain areas will plastically adapt their functional connectivity following injury may allow us to decipher why some brain lesions lead to permanent loss of cognitive function, while others do not.
Keywords: hippocampus; multimodal MRI; network analysis; neuroscience; neurotoxic lesions; non-human primates; plasticity; rhesus macaque.
© 2018, Froudist-Walsh et al.
Conflict of interest statement
SF, PB, JY, KM, RM, LF, PC No competing interests declared
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

