RAMP1 in Kupffer cells is a critical regulator in immune-mediated hepatitis
- PMID: 30462657
- PMCID: PMC6248891
- DOI: 10.1371/journal.pone.0200432
RAMP1 in Kupffer cells is a critical regulator in immune-mediated hepatitis
Abstract
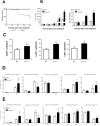
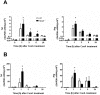
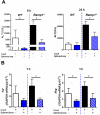
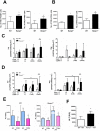
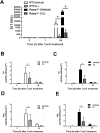
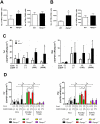
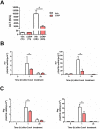
The significance of the relationship between the nervous and immune systems with respect to disease course is increasingly apparent. Immune cells in the liver and spleen are responsible for the development of acute liver injury, yet the regulatory mechanisms of the interactions remain elusive. Calcitonin gene-related peptide (CGRP), which is released from the sensory nervous system, regulates innate immune activation via receptor activity-modifying protein 1 (RAMP1), a subunit of the CGRP receptor. Here, we show that RAMP1 in Kupffer cells (KCs) plays a critical role in the etiology of immune-mediated hepatitis. RAMP1-deficient mice with concanavalin A (ConA)-mediated hepatitis, characterized by severe liver injury accompanied by infiltration of immune cells and increased secretion of pro-inflammatory cytokines by KCs and splenic T cells, showed poor survival. Removing KCs ameliorated liver damage, while depleting T cells or splenectomy led to partial amelioration. Adoptive transfer of splenic T cells from RAMP1-deficient mice led to a modest increase in liver injury. Co-culture of KCs with splenic T cells led to increased cytokine expression by both cells in a RAMP1-dependent manner. Thus, immune-mediated hepatitis develops via crosstalk between immune cells. RAMP1 in KCs is a key regulator of immune responses.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Heymann F, Tacke F. Immunology in the liver—from homeostasis to disease. Nat Rev Gastroenterol Hepatol. 2016. February;13(2):88–110. 10.1038/nrgastro.2015.200 Epub 2016 Jan 13. Review. . - DOI - PubMed
-
- Jensen KJ, Alpini G, Glaser S. Hepatic nervous system and neurobiology of the liver. Compr Physiol. 2013. April;3(2):655–65. 10.1002/cphy.c120018 Review. - DOI - PMC - PubMed
-
- Stoyanova II, Gulubova MV. Immunocytochemical study on the liver innervation in patients with cirrhosis. Acta Histochem. 2000. November;102(4):391–402. 10.1078/0065-1281-00568 . - DOI - PubMed
-
- Jung WC, Levesque JP, Ruitenberg MJ. It takes nerve to fight back: The significance of neural innervation of the bone marrow and spleen for immune function. Semin Cell Dev Biol. 2017. January;61:60–70. 10.1016/j.semcdb.2016.08.010 Epub 2016 Aug 11. Review. . - DOI - PubMed
-
- Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002. June;54(2):233–46. Review. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

