Aliphatic Radical Relay Heck Reaction at Unactivated C(sp3 )-H Sites of Alcohols
- PMID: 30462879
- PMCID: PMC6359925
- DOI: 10.1002/anie.201812398
Aliphatic Radical Relay Heck Reaction at Unactivated C(sp3 )-H Sites of Alcohols
Abstract
The Mizoroki-Heck reaction is one of the most efficient methods for alkenylation of aryl, vinyl, and alkyl halides. Given its innate nature, this protocol requires the employment of compounds possessing a halogen atom at the site of functionalization. However, the accessibility of organic molecules possessing a halogen atom at a particular site in aliphatic systems is extremely limited. Thus, a protocol that allows a Heck reaction to occur at a specific nonfunctionalized C(sp3 )-H site is desirable. Reported here is a radical relay Heck reaction which allows selective remote alkenylation of aliphatic alcohols at unactivated β-, γ-, and δ-C(sp3 )-H sites. The use of an easily installed/removed Si-based auxiliary enables selective I-atom/radical translocation events at remote C-H sites followed by the Heck reaction. Notably, the reaction proceeds smoothly under mild visible-light-mediated conditions at room temperature, producing highly modifiable and valuable alkenol products from readily available alcohols feedstocks.
Keywords: C−H activation; Heck reaction; palladium; photochemistry; radicals.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflict of interest
The authors declare no conflict of interest.
Figures
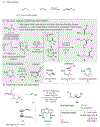



References
-
- Ma W, Gandeepan P, Li J, Ackermann L, Org. Chem. Front 2017, 4, 1435–1467.
-
- He J, Li S, Deng Y, Fu H, Laforteza BN, Spangler JE, Homs A, Yu J-Q, Science 2014, 343, 1216–1220; - PMC - PubMed
- Jiang H, He J, Liu T, Yu J-Q, J. Am. Chem. Soc 2016, 138, 2055–2059; - PMC - PubMed
- Stowers KJ, Fortner KC, Sanford MS, J. Am. Chem. Soc 2011, 133, 6541–6544; - PMC - PubMed
- He C, Gaunt MJ, Chem. Sci 2017, 8, 3586–3592; - PMC - PubMed
- Zhuang Z, Yu C-B, Chen G, Wu Q-F, Hsiao Y, Joe CL, Qiao JX, Poss MA, Yu J-Q, J. Am. Chem. Soc 2018, 140, 10363–10367. - PMC - PubMed
-
-
See Ref. [3b] and:
- Thrimurtulu N, Khan S, Maity S, Volla CMR, Maiti D, Chem. Commun 2017, 53, 12457–12460. - PubMed
-
-
- Oestreich M, The Mizoroki – Heck Reaction, Wiley, Chichester, 2009;
- Beletskaya IP, Cheprakov AV, Chem. Rev 2000, 100, 3009–3066. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

