Modulation of Host Immunity by Helminths: The Expanding Repertoire of Parasite Effector Molecules
- PMID: 30462997
- PMCID: PMC6269126
- DOI: 10.1016/j.immuni.2018.10.016
Modulation of Host Immunity by Helminths: The Expanding Repertoire of Parasite Effector Molecules
Abstract
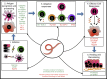
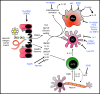
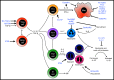
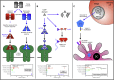
Helminths are extraordinarily successful parasites due to their ability to modulate the host immune response. They have evolved a spectrum of immunomodulatory molecules that are now beginning to be defined, heralding a molecular revolution in parasite immunology. These discoveries have the potential both to transform our understanding of parasite adaptation to the host and to develop possible therapies for immune-mediated disease. In this review we will summarize the current state of the art in parasite immunomodulation and discuss perspectives on future areas for research and discovery.
Copyright © 2018 Elsevier Inc. All rights reserved.
Figures
References
-
- Akdis M., Akdis C.A. Mechanisms of allergen-specific immunotherapy: multiple suppressor factors at work in immune tolerance to allergens. J. Allergy Clin. Immunol. 2014;133:621–631. - PubMed
-
- Al-Riyami L., Pineda M.A., Rzepecka J., Huggan J.K., Khalaf A.I., Suckling C.J., Scott F.J., Rodgers D.T., Harnett M.M., Harnett W. Designing anti-inflammatory drugs from parasitic worms: a synthetic small molecule analogue of the Acanthocheilonema viteae product ES-62 prevents development of collagen-induced arthritis. J. Med. Chem. 2013;56:9982–10002. - PMC - PubMed
-
- Alvarado R., To J., Lund M.E., Pinar A., Mansell A., Robinson M.W., O’Brien B.A., Dalton J.P., Donnelly S. The immune modulatory peptide FhHDM-1 secreted by the helminth Fasciola hepatica prevents NLRP3 inflammasome activation by inhibiting endolysosomal acidification in macrophages. FASEB J. 2017;31:85–95. - PubMed
-
- Ambadapadi S., Munuswamy-Ramanujam G., Zheng D., Sullivan C., Dai E., Morshed S., McFadden B., Feldman E., Pinard M., McKenna R. Reactive center loop (RCL) peptides derived from serpins display independent coagulation and immune modulating activities. J. Biol. Chem. 2016;291:2874–2887. - PMC - PubMed
-
- Anbu K.A., Joshi P. Identification of a 55 kDa Haemonchus contortus excretory/secretory glycoprotein as a neutrophil inhibitory factor. Parasite Immunol. 2008;30:23–30. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

