Heart Regeneration in the Mexican Cavefish
- PMID: 30462998
- PMCID: PMC6280125
- DOI: 10.1016/j.celrep.2018.10.072
Heart Regeneration in the Mexican Cavefish
Abstract
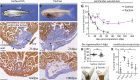
Although Astyanax mexicanus surface fish regenerate their hearts after injury, their Pachón cave-dwelling counterparts cannot and, instead, form a permanent fibrotic scar, similar to the human heart. Myocardial proliferation peaks at similar levels in both surface fish and Pachón 1 week after injury. However, in Pachón, this peak coincides with a strong scarring and immune response, and ultimately, cavefish cardiomyocytes fail to replace the scar. We identified lrrc10 to be upregulated in surface fish compared with Pachón after injury. Similar to cavefish, knockout of lrrc10 in zebrafish impairs heart regeneration without affecting wound cardiomyocyte proliferation. Furthermore, using quantitative trait locus (QTL) analysis, we have linked the degree of heart regeneration to three loci in the genome, identifying candidate genes fundamental to the difference between scarring and regeneration. Our study provides evidence that successful heart regeneration entails a delicate interplay between cardiomyocyte proliferation and scarring.
Keywords: Mexican cavefish; QTL; fibrotic scar; heart regeneration; lrrc10; myocardial proliferation.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Adameyko I.I., Mudry R.E., Houston-Cummings N.R.M., Veselov A.P., Gregorio C.C., Tevosian S.G. Expression and regulation of mouse SERDIN1, a highly conserved cardiac-specific leucine-rich repeat protein. Dev. Dyn. 2005;233:540–552. - PubMed
-
- Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. - PubMed
-
- Barrallo-Gimeno A., Nieto M.A. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- FS/14/73/31107/BHF_/British Heart Foundation/United Kingdom
- RG/18/5/33532/BHF_/British Heart Foundation/United Kingdom
- PG/15/111/31939/BHF_/British Heart Foundation/United Kingdom
- RG/13/9/30269/BHF_/British Heart Foundation/United Kingdom
- BB/C517041/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

