Needle-Free Immunization with Chitosan-Based Systems
- PMID: 30463211
- PMCID: PMC6274840
- DOI: 10.3390/ijms19113639
Needle-Free Immunization with Chitosan-Based Systems
Abstract
Despite successful use, needle-based immunizations have several issues such as the risk of injuries and infections from the reuse of needles and syringes and the low patient compliance due to pain and fear of needles during immunization. In contrast, needle-free immunizations have several advantages including ease of administration, high level of patient compliance and the possibility of mass vaccination. Thus, there is an increasing interest on developing effective needle-free immunizations via cutaneous and mucosal approaches. Here, we discuss several methods of needle-free immunizations and provide insights into promising use of chitosan systems for successful immunization.
Keywords: chitosan; mucosal vaccine; needle-free immunization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




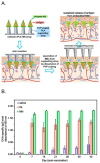

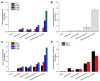
Similar articles
-
Immunization without needles.Nat Rev Immunol. 2005 Dec;5(12):905-16. doi: 10.1038/nri1728. Nat Rev Immunol. 2005. PMID: 16239901 Review.
-
Survey of the prevalence of immunization non-compliance due to needle fears in children and adults.Vaccine. 2012 Jul 6;30(32):4807-12. doi: 10.1016/j.vaccine.2012.05.011. Epub 2012 May 19. Vaccine. 2012. PMID: 22617633
-
[Cost of tetanus toxoid injection using a jet-injector (Imule) in collective immunization in Senegal: comparison with injection using a syringe and resterilizable needle].Sante. 1999 Sep-Oct;9(5):319-26. Sante. 1999. PMID: 10657777 French.
-
Needle-free vaccine delivery.Adv Drug Deliv Rev. 2006 Apr 20;58(1):68-89. doi: 10.1016/j.addr.2005.12.003. Epub 2006 Mar 24. Adv Drug Deliv Rev. 2006. PMID: 16564111 Review.
-
Needle size for vaccination procedures in children and adolescents.Cochrane Database Syst Rev. 2018 Aug 9;8(8):CD010720. doi: 10.1002/14651858.CD010720.pub3. Cochrane Database Syst Rev. 2018. PMID: 30091147 Free PMC article.
Cited by
-
Peptide-Based Vaccine against Breast Cancer: Recent Advances and Prospects.Pharmaceuticals (Basel). 2023 Jun 25;16(7):923. doi: 10.3390/ph16070923. Pharmaceuticals (Basel). 2023. PMID: 37513835 Free PMC article. Review.
-
Tumor microenvironment penetrating chitosan nanoparticles for elimination of cancer relapse and minimal residual disease.Front Oncol. 2022 Nov 30;12:1054029. doi: 10.3389/fonc.2022.1054029. eCollection 2022. Front Oncol. 2022. PMID: 36531004 Free PMC article. Review.
-
Evaluation of mucosal adjuvants to chitosan-nanoparticle-based oral subunit vaccine for controlling salmonellosis in broilers.Front Immunol. 2025 Feb 3;16:1509990. doi: 10.3389/fimmu.2025.1509990. eCollection 2025. Front Immunol. 2025. PMID: 39981235 Free PMC article.
-
Hyaluronic Acid Nanocapsules as a Platform for Needle-Free Vaccination.Pharmaceutics. 2019 May 26;11(5):246. doi: 10.3390/pharmaceutics11050246. Pharmaceutics. 2019. PMID: 31130688 Free PMC article.
-
Microarray patches enable the development of skin-targeted vaccines against COVID-19.Adv Drug Deliv Rev. 2021 Apr;171:164-186. doi: 10.1016/j.addr.2021.01.022. Epub 2021 Feb 2. Adv Drug Deliv Rev. 2021. PMID: 33539853 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

