The Effect of Small Doses of Fructose and Its Epimers on Glycemic Control: A Systematic Review and Meta-Analysis of Controlled Feeding Trials
- PMID: 30463314
- PMCID: PMC6266436
- DOI: 10.3390/nu10111805
The Effect of Small Doses of Fructose and Its Epimers on Glycemic Control: A Systematic Review and Meta-Analysis of Controlled Feeding Trials
Abstract
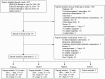
Objective: Contrary to the concerns that fructose may have adverse metabolic effects, an emerging literature has shown that small doses (≤10 g/meal) of fructose and its low-caloric epimers (allulose, tagatose, and sorbose) decrease the glycemic response to high glycemic index meals. Whether these acute reductions manifest as sustainable improvements in glycemic control is unclear. Our objective was to synthesize the evidence from controlled feeding trials that assessed the effect of small doses of fructose and its low-caloric epimers on glycemic control. Methods: We searched MEDLINE, EMBASE, and the Cochrane Library through April 18, 2018. We included controlled feeding trials of ≥1 week that investigated the effect of small doses (≤50 g/day or ≤10% of total energy intake/day) of fructose and its low-caloric epimers on HbA1c, fasting glucose, and fasting insulin. Two independent reviewers extracted data and assessed risk of bias. Data were pooled using the generic inverse variance method and expressed as mean differences (MDs) with 95% confidence intervals (CIs). Heterogeneity was assessed using the Cochran Q statistic and quantified using the I² statistic. Grading of Recommendations Assessment, Development and Evaluation (GRADE) assessed the certainty of the evidence. Results: We identified 14 trial comparisons (N = 337) of the effect of fructose in individuals with and without diabetes, 3 trial comparisons (N = 138) of the effect of allulose in individuals without diabetes, 3 trial comparisons (N = 376) of the effect of tagatose mainly in individuals with type 2 diabetes, and 0 trial comparisons of the effect of sorbose. Small doses of fructose and tagatose significantly reduced HbA1c (MD = -0.38% (95% CI: -0.64%, -0.13%); MD = -0.20% (95% CI: -0.34%, -0.06%)) and fasting glucose (MD = -0.13 mmol/L (95% CI: -0.24 mmol/L, -0.03 mmol/L)); MD = -0.30 mmol/L (95% CI: -0.57 mmol/L, -0.04 mmol/L)) without affecting fasting insulin (p > 0.05). Small doses of allulose did not have a significant effect on HbA1c and fasting insulin (p > 0.05), while the reduction in fasting glucose was of borderline significance (p = 0.05). The certainty of the evidence of the effect of small doses of fructose and allulose on HbA1c, fasting glucose, and fasting insulin was graded as low. The certainty of the evidence of the effect of tagatose on HbA1c, fasting glucose, and fasting insulin was graded as moderate. Conclusions: Our results indicate that small doses of fructose and tagatose may improve glycemic control over the long term. There is a need for long-term randomized controlled trials for all four sugars to improve our certainty in the estimates.
Keywords: HbA1c; allulose; catalytic dose; fructose; glucose; glycemia; insulin; meta-analysis; sorbose; tagatose.
Conflict of interest statement
J.C.N. has worked as a clinical research coordinator at Glycemic Index Laboratories, Toronto, Ontario, Canada. C.W.C.K. has received grant grants or research support from the Advanced Food Materials Network, Agriculture and Agri-Foods Canada (AAFC), Almond Board of California, American Pistachio Growers, Barilla, Calorie Control Council, Canadian Institutes of Health Research (CIHR), Canola Council of Canada, International Nut and Dried Fruit Council, International Tree Nut Council Research and Education Foundation, Loblaw Brands Ltd., Pulse Canada, Saskatchewan Pulse Growers, and Unilever. He has received in-kind research support from the Almond Board of California, American Peanut Council, Barilla, California Walnut Commission, Kellogg Canada, Loblaw Companies, Quaker (Pepsico), Primo, Unico, Unilever, and White Wave Foods. He has received travel support and/or honoraria from the American Peanut Council, American Pistachio Growers, Barilla, California Walnut Commission, Canola Council of Canada, General Mills, International Nut and Dried Fruit Council, International Pasta Organization, Loblaw Brands Ltd., Nutrition Foundation of Italy, Oldways Preservation Trust, Paramount Farms, Peanut Institute, Pulse Canada, Sabra Dipping Co., Saskatchewan Pulse Growers, Sun-Maid, Tate & Lyle, Unilever, and White Wave Foods. He has served on the scientific advisory board for the International Tree Nut Council, International Pasta Organization, McCormick Science Institute, Oldways Preservation Trust, Paramount Farms and Pulse Canada. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD), is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD and is a Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. T.M.S.W. is a part owner and the President of Glycemic Index Laboratories, Inc, Toronto, Canada, and has authored several popular diet books on the glycemic index for which he has received royalties from Phillipa Sandall Publishing Services and CABI Publishers. He has received consultant fees, honoraria, travel funding, or research support from or served on the scientific advisory board for CIHR, Diabetes Canada, Dairy Farmers of Canada, McCain Foods, Temasek Polytechnic, Northwestern University, Royal Society of London, Glycemic Index Symbol program, CreaNutrition AG, McMaster University, Canadian Society for Nutritional Sciences, National Sports and Conditioning Association, Faculty of Public Health and Nutrition—Autonomous University of Nuevo Leon, Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD). J.L.S. has received research support from the Canadian Institutes of health Research (CIHR), Diabetes Canada, PSI Foundation, Calorie Control Council, Banting and Best Diabetes Centre (BBDC), American Society for Nutrition (ASN), Dr. Pepper Snapple Group (investigator initiated, unrestricted donation), INC International Nut and Dried Fruit Council, and The Tate and Lyle Nutritional Research Fund at the University of Toronto. He has received speaker fees and/or honoraria from Diabetes Canada, Canadian Nutrition Society (CNS), University of Alabama at Birmingham, Abbott Laboratories, Canadian Sugar Institute, Dr. Pepper Snapple Group, The Coca-Cola Company, Dairy Farmers of Canada, Nutrition Foundation of Italy (NFI), C3 Collaborating for Health, WhiteWave Foods, Rippe Lifestyle, mdBriefcase, Alberta Milk, FoodMinds LLC, Memac Ogilvy & Mather LLC, PepsiCo, and Pulse Canada. He has ad hoc consulting arrangements with Winston & Strawn LLP, Perkins Coie LLP, and Tate & Lyle. He is a member of the European Fruit Juice Association Scientific Expert Panel. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), and Canadian Cardiovascular Society (CCS), as well as an expert writing panel of the American Society for Nutrition (ASN). He serves as an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of the International Life Science Institute (ILSI) North America. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His wife is an employee of Unilever Canada. No competing interests were declared by C.R.B., S.B.M., T.A.K., and L.A.L.
Figures

References
-
- Oshima H., Kimura I., Izumori K. Psicose Contents in Various Food Products and its Origin. Food Sci. Technol. Res. 2006;12:137–143. doi: 10.3136/fstr.12.137. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

