Epithelial-Mesenchymal Transition and Metastasis under the Control of Transforming Growth Factor β
- PMID: 30463358
- PMCID: PMC6274739
- DOI: 10.3390/ijms19113672
Epithelial-Mesenchymal Transition and Metastasis under the Control of Transforming Growth Factor β
Abstract
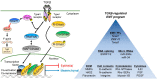
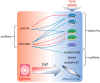
Metastasis of tumor cells from primary sites of malignancy to neighboring stromal tissue or distant localities entails in several instances, but not in every case, the epithelial-mesenchymal transition (EMT). EMT weakens the strong adhesion forces between differentiated epithelial cells so that carcinoma cells can achieve solitary or collective motility, which makes the EMT an intuitive mechanism for the initiation of tumor metastasis. EMT initiates after primary oncogenic events lead to secondary secretion of cytokines. The interaction between tumor-secreted cytokines and oncogenic stimuli facilitates EMT progression. A classic case of this mechanism is the cooperation between oncogenic Ras and the transforming growth factor β (TGFβ). The power of TGFβ to mediate EMT during metastasis depends on versatile signaling crosstalk and on the regulation of successive waves of expression of many other cytokines and the progressive remodeling of the extracellular matrix that facilitates motility through basement membranes. Since metastasis involves many organs in the body, whereas EMT affects carcinoma cell differentiation locally, it has frequently been debated whether EMT truly contributes to metastasis. Despite controversies, studies of circulating tumor cells, studies of acquired chemoresistance by metastatic cells, and several (but not all) metastatic animal models, support a link between EMT and metastasis, with TGFβ, often being a common denominator in this link. This article aims at discussing mechanistic cases where TGFβ signaling and EMT facilitate tumor cell dissemination.
Keywords: epithelial-mesenchymal transition; micro-RNA; non-coding RNA; signal transduction; transcription factor; transforming growth factor β; tumor invasiveness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

