The Plasma Membrane: A Platform for Intra- and Intercellular Redox Signaling
- PMID: 30463362
- PMCID: PMC6262572
- DOI: 10.3390/antiox7110168
The Plasma Membrane: A Platform for Intra- and Intercellular Redox Signaling
Abstract
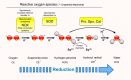
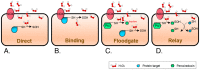
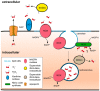
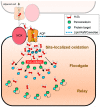
Membranes are of outmost importance to allow for specific signal transduction due to their ability to localize, amplify, and direct signals. However, due to the double-edged nature of reactive oxygen species (ROS)-toxic at high concentrations but essential signal molecules-subcellular localization of ROS-producing systems to the plasma membrane has been traditionally regarded as a protective strategy to defend cells from unwanted side-effects. Nevertheless, specialized regions, such as lipid rafts and caveolae, house and regulate the activated/inhibited states of important ROS-producing systems and concentrate redox targets, demonstrating that plasma membrane functions may go beyond acting as a securing lipid barrier. This is nicely evinced by nicotinamide adenine dinucleotide phosphate (NADPH)-oxidases (NOX), enzymes whose primary function is to generate ROS and which have been shown to reside in specific lipid compartments. In addition, membrane-inserted bidirectional H₂O₂-transporters modulate their conductance precisely during the passage of the molecules through the lipid bilayer, ensuring time-scaled delivery of the signal. This review aims to summarize current evidence supporting the role of the plasma membrane as an organizing center that serves as a platform for redox signal transmission, particularly NOX-driven, providing specificity at the same time that limits undesirable oxidative damage in case of malfunction. As an example of malfunction, we explore several pathological situations in which an inflammatory component is present, such as inflammatory bowel disease and neurodegenerative disorders, to illustrate how dysregulation of plasma-membrane-localized redox signaling impacts normal cell physiology.
Keywords: NADPH oxidase; aquaporin; inflammation; inflammatory bowel disease; lipid rafts; neurodegenerative disorders; plasma membrane; redox signaling; redoxosome.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Localizing NADPH oxidase-derived ROS.Sci STKE. 2006 Aug 22;2006(349):re8. doi: 10.1126/stke.3492006re8. Sci STKE. 2006. PMID: 16926363 Review.
-
Compartmentalization of redox signaling through NADPH oxidase-derived ROS.Antioxid Redox Signal. 2009 Jun;11(6):1289-99. doi: 10.1089/ars.2008.2333. Antioxid Redox Signal. 2009. PMID: 18999986 Free PMC article. Review.
-
The Importance of NADPH Oxidases and Redox Signaling in Angiogenesis.Antioxidants (Basel). 2017 May 13;6(2):32. doi: 10.3390/antiox6020032. Antioxidants (Basel). 2017. PMID: 28505091 Free PMC article. Review.
-
Soyasaponin Bb inhibits the recruitment of toll-like receptor 4 (TLR4) into lipid rafts and its signaling pathway by suppressing the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase-dependent generation of reactive oxygen species.Mol Nutr Food Res. 2016 Jul;60(7):1532-43. doi: 10.1002/mnfr.201600015. Epub 2016 Apr 21. Mol Nutr Food Res. 2016. PMID: 27005845
-
Redox-regulated growth factor survival signaling.Antioxid Redox Signal. 2013 Nov 20;19(15):1815-27. doi: 10.1089/ars.2012.5028. Epub 2013 Jan 15. Antioxid Redox Signal. 2013. PMID: 23198948 Review.
Cited by
-
Pulmonary hypertension and oxidative stress: Where is the link?Semin Fetal Neonatal Med. 2022 Aug;27(4):101347. doi: 10.1016/j.siny.2022.101347. Epub 2022 Apr 19. Semin Fetal Neonatal Med. 2022. PMID: 35473693 Free PMC article. Review.
-
Expression of CD70 Modulates Nitric Oxide and Redox Status in Endothelial Cells.Arterioscler Thromb Vasc Biol. 2022 Sep;42(9):1169-1185. doi: 10.1161/ATVBAHA.122.317866. Epub 2022 Aug 4. Arterioscler Thromb Vasc Biol. 2022. PMID: 35924558 Free PMC article.
-
Low-Intensity Pulsed Ultrasound Treatment Selectively Stimulates Senescent Cells to Promote SASP Factors for Immune Cell Recruitment.Aging Cell. 2025 May;24(5):e14486. doi: 10.1111/acel.14486. Epub 2025 Jan 16. Aging Cell. 2025. PMID: 39821933 Free PMC article.
-
Thermoprotection by a cell membrane-localized metacaspase in a green alga.Plant Cell. 2024 Feb 26;36(3):665-687. doi: 10.1093/plcell/koad289. Plant Cell. 2024. PMID: 37971931 Free PMC article.
-
Superoxide Radicals in the Execution of Cell Death.Antioxidants (Basel). 2022 Mar 4;11(3):501. doi: 10.3390/antiox11030501. Antioxidants (Basel). 2022. PMID: 35326151 Free PMC article. Review.
References
-
- Rall T.W., Sutherland E.W., Berthet J. The relationship of epinephrine and glucagon to liver phosphorylase. J. Biol. Chem. 1957;224:459–468. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

