YAP/TAZ Signaling as a Molecular Link between Fibrosis and Cancer
- PMID: 30463366
- PMCID: PMC6274979
- DOI: 10.3390/ijms19113674
YAP/TAZ Signaling as a Molecular Link between Fibrosis and Cancer
Abstract
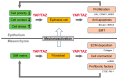
Tissue fibrosis is a pathological condition that is associated with impaired epithelial repair and excessive deposition of extracellular matrix (ECM). Fibrotic lesions increase the risk of cancer in various tissues, but the mechanism linking fibrosis and cancer is unclear. Yes-associated protein (YAP) and the transcriptional coactivator with PDZ-binding motif (TAZ) are core components of the Hippo pathway, which have multiple biological functions in the development, homeostasis, and regeneration of tissues and organs. YAP/TAZ act as sensors of the structural and mechanical features of the cell microenvironment. Recent studies have shown aberrant YAP/TAZ activation in both fibrosis and cancer in animal models and human tissues. In fibroblasts, ECM stiffness mechanoactivates YAP/TAZ, which promote the production of profibrotic mediators and ECM proteins. This results in tissue stiffness, thus establishing a feed-forward loop of fibroblast activation and tissue fibrosis. In contrast, in epithelial cells, YAP/TAZ are activated by the disruption of cell polarity and increased ECM stiffness in fibrotic tissues, which promotes the proliferation and survival of epithelial cells. YAP/TAZ are also involved in the epithelial⁻mesenchymal transition (EMT), which contributes to tumor progression and cancer stemness. Importantly, the crosstalk with transforming growth factor (TGF)-β signaling and Wnt signaling is essential for the profibrotic and tumorigenic roles of YAP/TAZ. In this article, we review the latest advances in the pathobiological roles of YAP/TAZ signaling and their function as a molecular link between fibrosis and cancer.
Keywords: Hippo pathway; TAZ; TGF-β; Wnt; YAP; cancer; fibrosis; mechanotransduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Lam A.P., Flozak A.S., Russell S., Wei J., Jain M., Mutlu G.M., Budinger G.R., Feghali-Bostwick C.A., Varga J., Gottardi C.J. Nuclear beta-catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am. J. Respir. Cell Mol. Boil. 2011;45:915–922. doi: 10.1165/rcmb.2010-0113OC. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

