Vaccination Guidelines for Patients With Immune-Mediated Disorders on Immunosuppressive Therapies
- PMID: 30463418
- PMCID: PMC6330697
- DOI: 10.1177/1203475418811335
Vaccination Guidelines for Patients With Immune-Mediated Disorders on Immunosuppressive Therapies
Abstract
Background:: Patients with immune-mediated diseases on immunosuppressive therapies have more infectious episodes than healthy individuals, yet vaccination practices by physicians for this patient population remain suboptimal.
Objectives:: To evaluate the safety and efficacy of vaccines in individuals exposed to immunosuppressive therapies and provide evidence-based clinical practice recommendations.
Methods:: A literature search for vaccination safety and efficacy in patients on immunosuppressive therapies (2009-2017) was conducted. Results were assessed using the Grading of Recommendation, Assessment, Development, and Evaluation system.
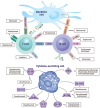
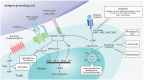
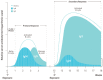
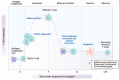
Results:: Several immunosuppressive therapies attenuate vaccine response. Thus, vaccines should be administered before treatment whenever feasible. Inactivated vaccines can be administered without treatment discontinuation. Similarly, evidence suggests that the live zoster vaccine is safe and effective while on select immunosuppressive therapy, although use of the subunit vaccine is preferred. Caution regarding other live vaccines is warranted. Drug pharmacokinetics, duration of vaccine-induced viremia, and immune response kinetics should be considered to determine appropriate timing of vaccination and treatment (re)initiation. Infants exposed to immunosuppressive therapies through breastmilk can usually be immunized according to local guidelines. Intrauterine exposure to immunosuppressive agents is not a contraindication for inactivated vaccines. Live attenuated vaccines scheduled for infants and children ⩾12 months of age, including measles, mumps, rubella, and varicella, can be safely administered as sufficient time has elapsed for drug clearance.
Conclusions:: Immunosuppressive agents may attenuate vaccine responses, but protective benefit is generally maintained. While these recommendations are evidence based, they do not replace clinical judgment, and decisions regarding vaccination must carefully assess the risks, benefits, and circumstances of individual patients.
Keywords: immune-mediated disease; immunosuppression; vaccination.
Conflict of interest statement
Figures
References
-
- Shigayeva A, Rudnick W, Green K, et al. Invasive pneumococcal disease among immunocompromised persons: implications for vaccination programs. Clin Infect Dis. 2016;62:139-147. - PubMed
-
- Doran MF, Crowson CS, Pond GR, O’Fallon WM, Gabriel SE. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum. 2002;46:2287-2293. - PubMed
-
- McKinnon JE, Maksimowicz-McKinnon K. Autoimmune disease and vaccination: impact on infectious disease prevention and a look at future applications. Transl Res. 2016;167:46-60. - PubMed
-
- Assala M, Groh M, Blanche P, et al. Pneumococcal and influenza vaccination rates in patients treated with corticosteroids and/or immunosuppressive therapies for systemic autoimmune diseases: a cross-sectional study. Joint Bone Spine. 2017;84:365-366. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

