Influence of viral infection on the relationships between airway cytokines and lung function in asthmatic children
- PMID: 30463560
- PMCID: PMC6249926
- DOI: 10.1186/s12931-018-0922-9
Influence of viral infection on the relationships between airway cytokines and lung function in asthmatic children
Abstract
Background: Few longitudinal studies examine inflammation and lung function in asthma. We sought to determine the cytokines that reduce airflow, and the influence of respiratory viral infections on these relationships.
Methods: Children underwent home collections of nasal lavage during scheduled surveillance periods and self-reported respiratory illnesses. We studied 53 children for one year, analyzing 392 surveillance samples and 203 samples from 85 respiratory illnesses. Generalized estimated equations were used to evaluate associations between nasal lavage biomarkers (7 mRNAs, 10 proteins), lung function and viral infection.
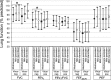
Results: As anticipated, viral infection was associated with increased cytokines and reduced FVC and FEV1. However, we found frequent and strong interactions between biomarkers and virus on lung function. For example, in the absence of viral infection, CXCL10 mRNA, MDA5 mRNA, CXCL10, IL-4, IL-13, CCL4, CCL5, CCL20 and CCL24 were negatively associated with FVC. In contrast, during infection, the opposite relationship was frequently found, with IL-4, IL-13, CCL5, CCL20 and CCL24 levels associated with less severe reductions in both FVC and FEV1.
Conclusions: In asthmatic children, airflow obstruction is driven by specific pro-inflammatory cytokines. In the absence of viral infection, higher cytokine levels are associated with decreasing lung function. However, with infection, there is a reversal in this relationship, with cytokine abundance associated with reduced lung function decline. While nasal samples may not reflect lower airway responses, these data suggest that some aspects of the inflammatory response may be protective against viral infection. This study may have ramifications for the treatment of viral-induced asthma exacerbations.
Keywords: Asthma; Chemokine; Children; Cytokine; FEV1; FVC; Rhinovirus; Urban; Viral.
Conflict of interest statement
Ethics approval and consent to participate
Research was performed in accordance with the Declaration of Helsinki (
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




References
-
- Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005-2009. Natl Health Stat Report. 2011;12:1–14. - PubMed
-
- Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

