Glial-neuronal signaling mechanisms underlying the neuroinflammatory effects of manganese
- PMID: 30463564
- PMCID: PMC6247759
- DOI: 10.1186/s12974-018-1349-4
Glial-neuronal signaling mechanisms underlying the neuroinflammatory effects of manganese
Abstract
Background: Exposure to increased manganese (Mn) causes inflammation and neuronal injury in the cortex and basal ganglia, resulting in neurological symptoms resembling Parkinson's disease. The mechanisms underlying neuronal death from exposure to Mn are not well understood but involve inflammatory activation of microglia and astrocytes. Expression of neurotoxic inflammatory genes in glia is highly regulated through the NF-κB pathway, but factors modulating neurotoxic glial-glial and glial-neuronal signaling by Mn are not well understood.
Methods: We examined the role of NF-κB in Mn-induced neurotoxicity by exposing purified microglia, astrocytes (from wild-type and astrocyte-specific IKK knockout mice), and mixed glial cultures to varying Mn concentrations and then treating neurons with the conditioned media (GCM) of each cell type. We hypothesized that mixed glial cultures exposed to Mn (0-100 μM) would enhance glial activation and neuronal death compared to microglia, wild-type astrocytes, or IKK-knockout astrocytes alone or in mixed cultures.
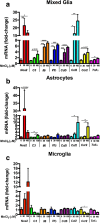
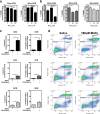
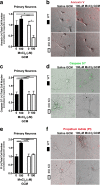
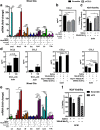
Results: Mixed glial cultures treated with 0-100 μM Mn for 24 h showed the most pronounced effect of increased expression of inflammatory genes including inducible nitric oxide synthase (Nos2), Tnf, Ccl5, Il6, Ccr2, Il1b, and the astrocyte-specific genes, C3 and Ccl2. Gene deletion of IKK2 in astrocytes dramatically reduced cytokine release in Mn-treated mixed glial cultures. Measurement of neuronal viability and apoptosis following exposure to Mn-GCM demonstrated that mixed glial cultures induced greater neuronal death than either cell type alone. Loss of IKK in astrocytes also decreased neuronal death compared to microglia alone, wild-type astrocytes, or mixed glia.
Conclusions: This suggests that astrocytes are a critical mediator of Mn neurotoxicity through enhanced expression of inflammatory cytokines and chemokines, including those most associated with a reactive phenotype such as CCL2 but not C3.
Keywords: Astrocyte; CCL2; Glial-glial communication; Glial-neuronal communication; Manganism; NF-κB; Neuroinflammation.
Conflict of interest statement
Authors’ information
Not applicable. Information presented on title page per the “instructions to authors.”
Ethics approval and consent to participate
All procedures were performed in accordance with National Institutes of Health guidelines for the care and use of laboratory animals with approval by the Institutional Animal Care and Use Committee of Colorado State University.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




Similar articles
-
Manganese-induced NF-kappaB activation and nitrosative stress is decreased by estrogen in juvenile mice.Toxicol Sci. 2011 Jul;122(1):121-33. doi: 10.1093/toxsci/kfr091. Epub 2011 Apr 21. Toxicol Sci. 2011. PMID: 21512103 Free PMC article.
-
Microglia-specific NF-κB signaling is a critical regulator of prion-induced glial inflammation and neuronal loss.PLoS Pathog. 2025 Jun 18;21(6):e1012582. doi: 10.1371/journal.ppat.1012582. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40532025 Free PMC article.
-
Microglia amplify inflammatory activation of astrocytes in manganese neurotoxicity.J Neuroinflammation. 2017 May 5;14(1):99. doi: 10.1186/s12974-017-0871-0. J Neuroinflammation. 2017. PMID: 28476157 Free PMC article.
-
Inflammatory Activation of Microglia and Astrocytes in Manganese Neurotoxicity.Adv Neurobiol. 2017;18:159-181. doi: 10.1007/978-3-319-60189-2_8. Adv Neurobiol. 2017. PMID: 28889267 Free PMC article. Review.
-
Role of glial cells in manganese neurotoxicity.J Appl Toxicol. 2012 May;32(5):310-7. doi: 10.1002/jat.1762. Epub 2011 Nov 26. J Appl Toxicol. 2012. PMID: 22120544 Review.
Cited by
-
Effect of metal ions on Alzheimer's disease.Brain Behav. 2022 Mar;12(3):e2527. doi: 10.1002/brb3.2527. Epub 2022 Feb 24. Brain Behav. 2022. PMID: 35212185 Free PMC article. Review.
-
The transcription factor REST up-regulates tyrosine hydroxylase and antiapoptotic genes and protects dopaminergic neurons against manganese toxicity.J Biol Chem. 2020 Mar 6;295(10):3040-3054. doi: 10.1074/jbc.RA119.011446. Epub 2020 Jan 30. J Biol Chem. 2020. PMID: 32001620 Free PMC article.
-
Role of manganese in brain health and disease: Focus on oxidative stress.Free Radic Biol Med. 2025 May;232:306-318. doi: 10.1016/j.freeradbiomed.2025.03.013. Epub 2025 Mar 12. Free Radic Biol Med. 2025. PMID: 40086492 Review.
-
Astrocyte inflammatory signaling mediates α-synuclein aggregation and dopaminergic neuronal loss following viral encephalitis.Exp Neurol. 2021 Dec;346:113845. doi: 10.1016/j.expneurol.2021.113845. Epub 2021 Aug 26. Exp Neurol. 2021. PMID: 34454938 Free PMC article.
-
Manganese exposure in juvenile C57BL/6 mice increases glial inflammatory responses in the substantia nigra following infection with H1N1 influenza virus.PLoS One. 2021 Jan 25;16(1):e0245171. doi: 10.1371/journal.pone.0245171. eCollection 2021. PLoS One. 2021. PMID: 33493177 Free PMC article.
References
-
- Riojas-Rodriguez H, Solis-Vivanco R, Schilmann A, Montes S, Rodriguez S, Rios C, Rodriguez-Agudelo Y. Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ Health Perspect. 2010;118(10):1465–1470. doi: 10.1289/ehp.0901229. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

