Nebulization versus standard application for topical anaesthesia during flexible bronchoscopy under moderate sedation - a randomized controlled trial
- PMID: 30463577
- PMCID: PMC6249909
- DOI: 10.1186/s12931-018-0926-5
Nebulization versus standard application for topical anaesthesia during flexible bronchoscopy under moderate sedation - a randomized controlled trial
Abstract
Background: Endobronchial administration of lidocaine is commonly used for cough suppression during diagnostic bronchoscopy. Recently, nebulization of lidocaine during bronchoscopies under deep sedation with fiberoptic intubation using a distinct spray catheter has been shown to have several advantages over conventional lidocaine administration via syringe. However, there are no data about this approach in bronchoscopies performed under moderate sedation. Therefore, this study compared the tolerability and safety of nebulized lidocaine with conventional lidocaine administration via syringe in patients undergoing bronchoscopy with moderate sedation.
Methods: Patients requiring diagnostic bronchoscopy were randomly assigned to receive topical lidocaine either via syringe or via nebulizer. Endpoints were consumption of lidocaine and sedative drugs, as well as patient tolerance and safety.
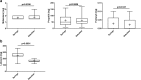
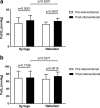
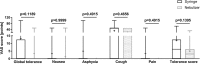
Results: Sixty patients were included in the study (n = 30 in each group). Patients required lower doses of endobronchial lidocaine when given via nebulizer versus syringe (164.7 ± 20.8 mg vs. 250.4 ± 42.38 mg; p < 0.0001) whereas no differences in the dosage of sedative drugs were observed between the two groups (all p > 0.05). Patients in the nebulizer group had higher mean oxygen saturation (96.19 ± 2.45% vs. 94.21 ± 3.02%; p = 0.0072) and a lower complication rate (0.3 ± 0.79 vs. 1.17 ± 1.62 per procedure; p = 0.0121) compared with those in the syringe group.
Conclusions: Endobronchial lidocaine administration via nebulizer was well-tolerated during bronchoscopies under moderate sedation and was associated with reduced lidocaine consumption, a lower complication rate and better oxygenation compared with lidocaine administration via syringe.
Trial registration: The study was registered with clinicaltrials.gov ( NCT02262442 ; 13th October 2014).
Keywords: Anaesthesia; Bronchoscopy; Lidocaine; Nebulizers and vaporizers.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol was approved by the Institutional Review Board for Human Studies at RWTH University, Aachen, Germany (14–074), and was performed in accordance with the ethical standards laid down in the Declaration of Helsinki. Written informed consent was obtained from all patients prior to inclusion into the study.
Consent for publication
Not applicable.
Competing interests
A discount was given for the purchase of the ENK fiberoptic atomizer from Cook medical. The funder had no role in planning or carrying out of the study. Apart from that the authors have no conflict of interest to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Du Rand IA, Blaikley J, Booton R, Chaudhuri N, Gupta V, Khalid S, Mandal S, Martin J, Mills J, Navani N, Rahman NM, Wrightson JM, Munavvar M, Group BTSBG British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax. 2013;68(Suppl 1):i1–i44. doi: 10.1136/thoraxjnl-2013-203618. - DOI - PubMed
-
- Wahidi MM, Jain P, Jantz M, Lee P, Mackensen GB, Barbour SY, Lamb C, Silvestri GA. American College of Chest Physicians consensus statement on the use of topical anesthesia, analgesia, and sedation during flexible bronchoscopy in adult patients. Chest. 2011;140:1342–1350. doi: 10.1378/chest.10-3361. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

