Therapeutic efficiency of human amniotic epithelial stem cell-derived functional hepatocyte-like cells in mice with acute hepatic failure
- PMID: 30463600
- PMCID: PMC6249765
- DOI: 10.1186/s13287-018-1063-2
Therapeutic efficiency of human amniotic epithelial stem cell-derived functional hepatocyte-like cells in mice with acute hepatic failure
Abstract
Background: Hepatocyte transplantation has been proposed as an effective treatment for patients with acute liver failure (ALF), but its application is limited by a severe shortage of donor livers. Human pluripotent stem cells (hPSCs) have emerged as a potential cell source for regenerative medicine. Human amniotic epithelial stem cells (hAESCs) derived from amniotic membrane have multilineage differentiation potential which makes them suitable for possible application in hepatocyte regeneration and ALF treatment.
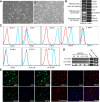
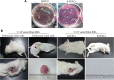
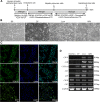
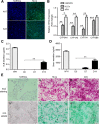
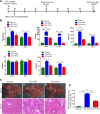
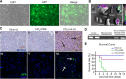
Methods: The pluripotent characteristics, immunogenicity, and tumorigenicity of hAESCs were studied by various methods. hAESCs were differentiated to hepatocyte-like cells (HLCs) using a non-transgenic and three-step induction protocol. ALB secretion, urea production, periodic acid-Schiff staining, and ICG uptake were performed to investigate the function of HLCs. The HLCs were transplanted into ALF NOD-SCID (nonobese diabetic severe combined immunodeficient) mouse, and the therapeutic effects were determined via liver function test, histopathology, and survival rate analysis. The ability of HLCs to engraft the damaged liver was evaluated by detecting the presence of GFP-positive cells.
Results: hAESCs expressed various markers of embryonic stem cells, epithelial stem cells, and mesenchymal stem cells and have low immunogenicity and no tumorigenicity. hAESC-derived hepatocytes possess the similar functions of human primary hepatocytes (hPH) such as producing urea, secreting ALB, uptaking ICG, storing glycogen, and expressing CYP enzymes. HLC transplantation via the tail vein could engraft in live parenchymal, improve the liver function, and protect hepatic injury from CCl4-induced ALF in mice. More importantly, HLC transplantation was able to significantly prolong the survival of ALF mouse.
Conclusion: We have established a rapid and efficient differentiation protocol that is able to successfully generate ample functional HLCs from hAESCs, in which the liver injuries and death rate of CCl4-induced ALF mouse can be significantly rescued by HLC transplantation. Therefore, our results may offer a superior approach for treating ALF.
Keywords: Acute liver failure; Cell transplantation; Hepatocyte-like cells; Human amniotic epithelial stem cells; Human primary hepatocytes.
Conflict of interest statement
Ethics approval
All procedures involving animals were approved by the Institutional Animal Care and Use Committees at Nanchang University and conducted in accordance with the national guidelines on animal care.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Human umbilical cord matrix stem cells efficiently rescue acute liver failure through paracrine effects rather than hepatic differentiation.Tissue Eng Part A. 2012 Jul;18(13-14):1352-64. doi: 10.1089/ten.TEA.2011.0516. Epub 2012 Jun 5. Tissue Eng Part A. 2012. PMID: 22519429 Free PMC article.
-
Differentiation and transplantation of human induced pluripotent stem cell-derived hepatocyte-like cells.Stem Cell Rev Rep. 2013 Aug;9(4):493-504. doi: 10.1007/s12015-011-9330-y. Stem Cell Rev Rep. 2013. PMID: 22076752
-
Human hepatic stem cells transplanted into a fulminant hepatic failure Alb-TRECK/SCID mouse model exhibit liver reconstitution and drug metabolism capabilities.Stem Cell Res Ther. 2015 Mar 26;6(1):49. doi: 10.1186/s13287-015-0038-9. Stem Cell Res Ther. 2015. PMID: 25889844 Free PMC article.
-
Hepatocyte transplantation and advancements in alternative cell sources for liver-based regenerative medicine.J Mol Med (Berl). 2018 Jun;96(6):469-481. doi: 10.1007/s00109-018-1638-5. Epub 2018 Apr 24. J Mol Med (Berl). 2018. PMID: 29691598 Free PMC article. Review.
-
Clinical Application of Pluripotent Stem Cells: An Alternative Cell-Based Therapy for Treating Liver Diseases?Transplantation. 2016 Dec;100(12):2548-2557. doi: 10.1097/TP.0000000000001426. Transplantation. 2016. PMID: 27495745 Review.
Cited by
-
Treatment of α-1 antitrypsin deficiency using hepatic-specified cells derived from human-induced pluripotent stem cells.Am J Transl Res. 2021 Apr 15;13(4):2710-2716. eCollection 2021. Am J Transl Res. 2021. PMID: 34017432 Free PMC article.
-
Human amniotic epithelial stem cells, a potential therapeutic approach for diabetes and its related complications.Hum Cell. 2025 Jan 3;38(2):39. doi: 10.1007/s13577-024-01171-x. Hum Cell. 2025. PMID: 39753919 Review.
-
A Survey and Critical Evaluation of Isolation, Culture, and Cryopreservation Methods of Human Amniotic Epithelial Cells.Cell Cycle. 2022 Apr;21(7):655-673. doi: 10.1080/15384101.2021.2020015. Epub 2022 Mar 15. Cell Cycle. 2022. PMID: 35289707 Free PMC article. Review.
-
Transcriptomic and computational analysis identified LPA metabolism, KLHL14 and KCNE3 as novel regulators of Epithelial-Mesenchymal Transition.Sci Rep. 2020 Mar 6;10(1):4180. doi: 10.1038/s41598-020-61017-y. Sci Rep. 2020. PMID: 32144311 Free PMC article.
-
Characteristics and Therapeutic Potential of Human Amnion-Derived Stem Cells.Int J Mol Sci. 2021 Jan 19;22(2):970. doi: 10.3390/ijms22020970. Int J Mol Sci. 2021. PMID: 33478081 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

