Impact of timing of continuous intravenous anesthetic drug treatment on outcome in refractory status epilepticus
- PMID: 30463604
- PMCID: PMC6249897
- DOI: 10.1186/s13054-018-2235-2
Impact of timing of continuous intravenous anesthetic drug treatment on outcome in refractory status epilepticus
Abstract
Background: Patients in refractory status epilepticus (RSE) may require treatment with continuous intravenous anesthetic drugs (cIVADs) for seizure control. The use of cIVADs, however, was recently associated with poor outcome in status epilepticus (SE), raising the question of whether cIVAD therapy should be delayed for attempts to halt seizures with repeated non-anesthetic antiepileptic drugs. In this study, we aimed to determine the impact of differences in therapeutic approaches on RSE outcome using timing of cIVAD therapy as a surrogate for treatment aggressiveness.
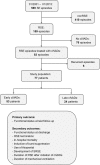
Methods: This was a retrospective cohort study over 14 years (n = 77) comparing patients with RSE treated with cIVADs within and after 48 h after RSE onset, and functional status at last follow-up was the primary outcome (good = return to premorbid baseline or modified Rankin Scale score of less than 3). Secondary outcomes included discharge functional status, in-hospital mortality, RSE termination, induction of burst suppression, use of thiopental, duration of RSE after initiation of cIVADs, duration of mechanical ventilation, and occurrence of super-refractory SE. Analysis was performed on the total cohort and on subgroups defined by RSE severity according to the Status Epilepticus Severity Score (STESS) and by the variables contained therein.
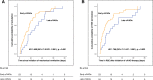
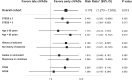
Results: Fifty-three (68.8%) patients received cIVADs within the first 48 h. Early cIVAD treatment was independently associated with good outcome (adjusted risk ratio [aRR] 3.175, 95% confidence interval [CI] 1.273-7.918; P = 0.013) as well as lower chance of both induction of burst suppression (aRR 0.661, 95% CI 0.507-0.861; P = 0.002) and use of thiopental (aRR 0.446, 95% CI 0.205-0.874; P = 0.043). RSE duration after cIVAD initiation was shorter in the early cIVAD cohort (hazard ratio 1.796, 95% CI 1.047-3.081; P = 0.033). Timing of cIVAD use did not impact the remaining secondary outcomes. Subgroup analysis revealed early cIVAD impact on the primary outcome to be driven by patients with STESS of less than 3.
Conclusions: Patients with RSE treated with cIVADs may benefit from early initiation of such therapy.
Keywords: Anesthetics; Continuous intravenous anesthetic drugs; Outcome; Refractory status epilepticus; Status Epilepticus Severity Score.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the local ethics committee of the Friedrich-Alexander University Erlangen-Nürnberg (FAU), Germany (vote number: 48_15Bc). Given the retrospective nature of the study, the need for informed consent was waived.
Consent for publication
Not applicable.
Competing interests
HBH and DM received unrestricted grants from UCB Pharma. HMH received grants from the EU and served on the scientific advisory boards of Cerbomed, Desitin, Eisai, Bial, and UCB Pharma. He received personal fees from IQWiG. He served on the speakers’ bureau of or received unrestricted grants from Desitin, Eisai, Novartis, Bial, Hexal, Boehringer Ingelheim, and UCB Pharma. CR, AGJ, TB, MIS, and RUK declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Treatment of Refractory Status Epilepticus With Continuous Intravenous Anesthetic Drugs: A Systematic Review.JAMA Neurol. 2024 May 1;81(5):534-548. doi: 10.1001/jamaneurol.2024.0108. JAMA Neurol. 2024. PMID: 38466294
-
Spectrum and Predictors of Refractory Status Epilepticus in a Developing Country.Can J Neurol Sci. 2017 Sep;44(5):538-546. doi: 10.1017/cjn.2017.28. Epub 2017 Apr 27. Can J Neurol Sci. 2017. PMID: 28446263
-
Features affecting treatment decisions and outcome in refractory status epilepticus.Epilepsia. 2025 Aug;66(8):2779-2789. doi: 10.1111/epi.18423. Epub 2025 Apr 22. Epilepsia. 2025. PMID: 40261726
-
Induction of burst suppression or coma using intravenous anesthetics in refractory status epilepticus.J Clin Neurosci. 2015 May;22(5):854-8. doi: 10.1016/j.jocn.2014.11.007. Epub 2015 Mar 3. J Clin Neurosci. 2015. PMID: 25744078
-
Status epilepticus: Refractory and super-refractory.Neurol India. 2017;65(Supplement):S12-S17. doi: 10.4103/neuroindia.NI_958_16. Neurol India. 2017. PMID: 28281491 Review.
Cited by
-
[Neurological intensive care medicine : Intensive medical care studies from 2018-2019].Anaesthesist. 2020 Feb;69(2):129-136. doi: 10.1007/s00101-019-00643-2. Anaesthesist. 2020. PMID: 31478057 German. No abstract available.
-
Status epilepticus in the ICU.Intensive Care Med. 2024 Jan;50(1):1-16. doi: 10.1007/s00134-023-07263-w. Epub 2023 Dec 20. Intensive Care Med. 2024. PMID: 38117319 Review.
-
Clinical characteristics of 27 children with febrile infection-related epilepsy syndrome in a single center.Pediatr Discov. 2024 Jun 9;2(2):e84. doi: 10.1002/pdi3.84. eCollection 2024 Jun. Pediatr Discov. 2024. PMID: 40625895 Free PMC article.
-
Treatment of acetylcholinesterase inhibitor-induced seizures with polytherapy targeting GABA and glutamate receptors.Neuropharmacology. 2021 Mar 1;185:108444. doi: 10.1016/j.neuropharm.2020.108444. Epub 2021 Jan 5. Neuropharmacology. 2021. PMID: 33359073 Free PMC article.
-
Epidemiology and Outcomes of Status Epilepticus.Int J Gen Med. 2021 Jun 28;14:2965-2973. doi: 10.2147/IJGM.S295855. eCollection 2021. Int J Gen Med. 2021. PMID: 34234526 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

